Abstract
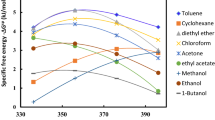
The investigations of interactions between polyolefins and test solutes at temperatures 58–122°C were carried out in the work. The test solutes were intentionally selected as representatives of the most important groups of compounds occurring in technological oils, which may be used as additives in conditions of industrial decomposition of polyolefins in Poland. For this purpose both the Flory-Huggins theory and inverse gas chromatography (IGC) were used. On the basis of retention data the values of both interaction and solubility parameters of analyzed polymers were determined. Solubility parameter δ and interaction parameter χ are related to some heat quantities e.g. excess free energy of mixing. It was observed influence of molecular mass and existence of chain branches on the values of the parameters. The obtained values allowed determination of influence of composition change of typical technological oils on their interactions with polymers and, at the same time, on course of charge preparation in these processes.
Similar content being viewed by others
References
A. G. Buekens and H. Huang, Resources, Conservation and Recycling, 23 (1998) 163.
M. J. Cran, S. W. Bigger and J. Scheirs, J. Therm. Anal. Cal., 81 (2005) 321.
F. S. M. Sinfrônio, J. C. O. Santos, L. G. Pereira, A. G. Souza, M. M. Conceição, V. J. Fernandes Jr. and V. M. Fonseca, J. Therm. Anal. Cal., 79 (2005) 393.
P. Straka and J. Náhunková, J. Therm. Anal. Cal., 76 (2004) 49.
P. Carniti and A. Gervasini, Thermochim. Acta, 379 (2001) 51.
J. Walendziewski, Fuel, 81 (2002) 473.
J. Walendziewski and M. Steininger, Catal. Today, 65 (2001) 323.
A. Tokarska, Z. N. Wydziału Budownictwa i Inżynierii Środowiska Politechniki Koszalińskiej, 21 (2003) 297 (in Polish).
A. Mianowski and T. Siudyga, J. Therm. Anal. Cal., 74 (2003) 623.
A. Mianowski and T. Siudyga, Z. N. Wydziału Budownictwa i Inżynierii Środowiska Politechniki Koszalińskiej, 21 (2003) 285 (In Polish).
T. Kamo, Y. Kondo, Y. Kodera, Y. Sato and S. Kushiyama, Fuel, 81 (2003) 187.
T. Durusoy, J. Therm. Anal. Cal., 79 (2005) 663.
A. Mianowski, P. Kałyniak and T. Siudyga, Diesel fuel from waste plastics, 5th IDENTIPLAST 2005, the Biennal Conference on the Recycling and Recovery of Plastics: Identifying the Opportunities for Plastics Recovery, 18–19.04.2005, Brussels, Belgium (electronic version).
G. DiPaola-Baranyi and J. E. Guillet, Macromolecules, 11 (1978) 228.
D. W. Van Krevelen, Coal. Topology-physics-chemistry-constitution, Elsevier 1993, pp. 485–487.
C. Etxabarren, M. Iriarte, C. Uriarte, A. Etxeberria and J. J. Iruin, J. Chromatogr. A, 969 (2002) 245.
D. Patterson, Y. B. Tewari, H. P. Schreiber and J. E. Guillet, Macromolecules, 4 (1971) 356.
A. Voelkel, Crit. Rev. Anal. Chem., 22 (1991) 411.
D. W. Van Krevelen, Fuel, 44 (1965) 229.
A. Voelkel and J. Fall, J. Chromatogr. A, 721 (1995) 139.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siudyga, T., Mianowski, A. Examination of polyolefins-organic compounds interactions by inverse gas chromatography. J Therm Anal Calorim 89, 191–196 (2007). https://doi.org/10.1007/s10973-006-7516-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-006-7516-3