Abstract
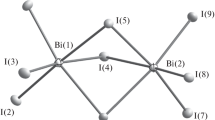
The thiourea complexes of antimony and bismuth triiodide were synthesized by a direct reaction of antimony and bismuth triiodide with thiourea powder at room temperature. The formula of the complex is MI3[SC(NH2)2]3(M=Sb, Bi). The crystal structure of the complexes belongs to monoclinic system and the lattice parameters are a=1.4772 nm, b=1.6582 nm, c=2.0674 nm and β=90.81° for SbI3(SC(NH2)2)3 and a=1.4009 nm, b=2.0170 nm, c=2.0397 nm and β=90.84° for BiI3[SC(NH2)2]3. The infrared spectra reveal that the trivalent antimony or bismuth ion is coordinated by the nitrogen atom, not the sulfur atom of the thiourea. Thermal analysis shows that there are two times structure rearrangements or phase transformation in the complexes from 100 to 170°C.
Similar content being viewed by others
References
H Oki K Otsuka (1976) Bull. Chem. Soc. Jpn. 49 1841 Occurrence Handle10.1246/bcsj.49.1841 Occurrence Handle1:CAS:528:DyaE28XlsFehsL8%3D
RA Siddiqui P Raj AK Saxena SK Dixit (1996) Synth. Reac. Inorg. Met.-org. Chem. 26 1189 Occurrence Handle1:CAS:528:DyaK28XkslChsrg%3D
GR Willey LT Daly PP Meehan MGB Drew (1996) J. Chem. Soc. Dalton Trans. 1 4045 Occurrence Handle10.1039/dt9960004045
MS Singh KP Rao (1999) Synth. Reac. Inorg. Met.-org. Chem. 29 541 Occurrence Handle1:CAS:528:DyaK1MXis12gs7k%3D
G Cantos CL Barbieri M Iacomini PAJ Gorin LR Travassos (1993) Biochem. J. 289 155 Occurrence Handle1:CAS:528:DyaK3sXmsVSqsbo%3D
J Kaloustian AM Pauli G Pieroni H Portugal (2002) J. Therm. Anal. Cal. 70 963 Occurrence Handle10.1023/A:1022233026068
L Xi L Yi W Jun L Huigang Q Songsheng (2002) J. Therm. Anal. Cal. 67 589 Occurrence Handle10.1023/A:1014392418707
N. N. Greenwood and A. Earnshaw, Chemistry of the Elements (2nd Ed.), Reed Educational and Professional Publishing Ltd. (1997) p. 553.
YC Guo SR Luan YR Chen XS Zang YQ Jia GQ Zhong SK Ruan (2002) J. Therm. Anal. Cal. 68 1025 Occurrence Handle10.1023/A:1016163111068 Occurrence Handle1:CAS:528:DC%2BD38XkvF2htbc%3D
RR Jia YX Yang YR Chen YQ Jia (2004) J. Therm. Anal. Cal. 76 157 Occurrence Handle10.1023/B:JTAN.0000027815.73030.29 Occurrence Handle1:CAS:528:DC%2BD2cXjvFSku7Y%3D
A Yamaguchi RP Penland S Mizushima TJ Lane C Curran JV Quagliano (1958) J. Am. Chem. Soc. 80 527 Occurrence Handle10.1021/ja01536a005 Occurrence Handle1:CAS:528:DyaG1cXjvVygtg%3D%3D
R Rivest (1962) Can. J. Chem. 40 2234 Occurrence Handle10.1139/v62-346 Occurrence Handle1:CAS:528:DyaF3sXht1ymuw%3D%3D
K Nakamoto et al. (1978) Infrared and Raman Spectra of Inorganic and Coordination Compounds (3rd Ed.) John Wiley & Sons Inc. New York
DM Adams JB Cornell (1967) J. Chem. Soc.(A) 1 884
RD Shannon (1976) Acta Crystallogr, .Sect. A. 32 751 Occurrence Handle10.1107/S0567739476001551
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhong, G.Q., Luan, S.R., Wang, P. et al. Synthesis, characterization and thermal decomposition of thiourea complexes of antimony and bismuth triiodide. J Therm Anal Calorim 86, 775–781 (2006). https://doi.org/10.1007/s10973-005-6959-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-6959-2