Abstract
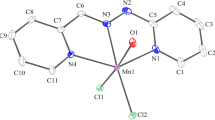
New divalent transition metal 3,5-pyrazoledicarboxylate hydrates of empirical formula Mpz(COO)2(H2O)2, where M=Mn, Co, Ni, Cu, Zn and Cd (pz(COO)2=3,5-pyrazoledicarboxylate), metal hydrazine complexes of the type Mpz(COO)2N2H4 where M=Co, Zn or Cd and Mpz(COO)2nN2H4·H2O, where n=1 for M=Ni and n=0.5 for M=Cu have been prepared and characterized by physico-chemical methods. Electronic spectroscopic data suggest that Co and Ni complexes adopt an octahedral geometry. The IR spectra confirm the presence of unidentate carboxylate anion (Δν=νasy(COO–)–νsym(COO–)>215 cm–1) in all the complexes and bidentate bridging hydrazine (νN–N=985–950 cm–1) in the metal hydrazine complexes. Both metal carboxylate and metal hydrazine carboxylate complexes undergo endothermic dehydration and/or dehydrazination followed by exothermic decomposition of organic moiety to give the respective metal oxides as the end products except manganese pyrazoledicarboxylate hydrate, which leaves manganese carbonate. X-ray powder diffraction patterns reveal that the metal carboxylate hydrates are isomorphous as are those of metal hydrazine complexes of cobalt, zinc and cadmium.
Similar content being viewed by others
References
EW Schmidt et al. (1984) Hydrazine and its Derivatives – Preparation, Properties and Applications Wiley Interscience New York
BT Heaton C Jacob P Page (1996) Coord. Chem. Rev. 154 193 Occurrence Handle10.1016/0010-8545(96)01285-4 Occurrence Handle1:CAS:528:DyaK28XlvFGmtLY%3D
A Ferrari A Braibanti G Bigliardi AM Lanfredi A Tiripicchio (1966) Nature 211 1174
A Braibanti F Dallavalle MA Pellinghelli E Laporati (1968) Inorg. Chem. 7 1430 Occurrence Handle10.1021/ic50065a034 Occurrence Handle1:CAS:528:DyaF1cXktl2qtrY%3D
BN Sivasankar S Govindarajan (1994) Thermochim. Acta 244 235 Occurrence Handle10.1016/0040-6031(94)80222-X Occurrence Handle1:CAS:528:DyaK2cXntFCntLk%3D
P Ravindranathan GV Mahesh KC Patil (1987) J. Solid State Chem. 66 20 Occurrence Handle10.1016/0022-4596(87)90216-7 Occurrence Handle1:CAS:528:DyaL2sXht1Kgur0%3D
BN Sivasankar S Govindarajan (1996) Mater. Res. Bull. 31 47 Occurrence Handle10.1016/0025-5408(95)00159-X Occurrence Handle1:CAS:528:DyaK28XhvFKitw%3D%3D
T Premkumar S Govindarajan (2003) Inorg. Chem. Commun. 6 1385 Occurrence Handle10.1016/j.inoche.2003.08.025 Occurrence Handle1:CAS:528:DC%2BD3sXnvVWqtbw%3D
GB Barlin et al. (1982) Heterocyclic Compounds, Vol. 41, The pyrazines Interscience New York Ch. IX
JC Bayon P Esteban G Net PG Rasmussen KN Bajer CW Hahn MM Gumz (1991) Inorg. Chem. 30 2572 Occurrence Handle10.1021/ic00011a023 Occurrence Handle1:CAS:528:DyaK3MXisFyntb0%3D
G Net JC Bayon WM Butler P Rasmussen (1989) J. Chem. Soc., Chem. Commun. 1 1022 Occurrence Handle10.1039/c39890001022
T Premkumar S Govindarajan (2002) Thermochim. Acta 386 35 Occurrence Handle10.1016/S0040-6031(01)00756-0 Occurrence Handle1:CAS:528:DC%2BD38XitFeqsL8%3D
T Premkumar S Govindarajan W-P Pan R-C Xie (2003) J. Therm. Anal. Cal. 74 325 Occurrence Handle10.1023/A:1026314911438 Occurrence Handle1:CAS:528:DC%2BD3sXosVGgt7w%3D
CW Hahn PG Rasmussen JC Bayon (1992) Inorg. Chem. 31 1963 Occurrence Handle10.1021/ic00036a046 Occurrence Handle1:CAS:528:DyaK38XisVOhtLk%3D
M Nakahanada K Ino S Kaizaki (1993) J. Chem. Soc., Dalton Trans. 1 3681 Occurrence Handle10.1039/dt9930003681
N Sakagami-Yoshida M Teramoto A Hioki A Fuyuhiro S Kaizaki (2000) Inorg. Chem. 39 5717 Occurrence Handle10.1021/ic000361f Occurrence Handle1:CAS:528:DC%2BD3cXotFeqsb0%3D
AI Vogel et al. (1986) A Text Book of Quantitative Inorganic Analysis, 4th Ed. Longman London
D Sanna G Micera P Buglyo T Kiss T Gajda P Surdy (1998) Inorg. Chim. Acta 268 297 Occurrence Handle10.1016/S0020-1693(97)05761-7 Occurrence Handle1:CAS:528:DyaK2sXotFWkurs%3D
ABP Lever et al. (1984) Inorganic Electronic Spectroscopy, 2nd Ed. Elsevier Amsterdam
K Nakamoto et al. (1978) Infrared and Raman Spectra of Inorganic and Coordination Compounds, 3rd Edn. Wiley Interscience New York
S Govindarajan KC Patil H Manohar PE Werner (1986) J. Chem. Soc., Dalton Trans. 1 119 Occurrence Handle10.1039/dt9860000119
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Premkumar, T., Govindarajan, S. Divalent transition metal complexes of 3,5-pyrazoledicarboxylate. J Therm Anal Calorim 84, 395–399 (2006). https://doi.org/10.1007/s10973-005-6932-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-6932-0