Summary
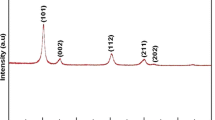
A homogeneous ZrO2 gel was obtained by hydrolysis-condensation of zirconium(IV) n-propoxide previously reacted with acetic acid. Dried zirconia powders were characterized by Fourier transformed infra-red (FTIR) and X-ray diffraction (XRD) analyses. Thermogravimetric (TG) and differential thermal analysis (DTA) coupled with mass spectrometric (MS) and gas chromatographic (GC) measurements were carried out in order to identify and quantify the organic products released during the ZrO2 gel pyrolysis. The TG-MS semi-quantitative analysis of the main released species allowed to describe the chemical rearrangement occurring in the solid during heating and to determine the chemical composition of the initial gel.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Egger, P., Dirè, S., Ischia, M. et al. Pyrolysis study of sol-gel derived zirconia by TG-GC-MS. J Therm Anal Calorim 81, 407–415 (2005). https://doi.org/10.1007/s10973-005-0800-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-0800-9