Abstract
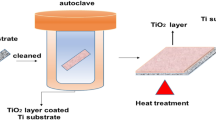
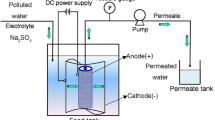
Ce-doped MnOx/Ti electrocatalytic membrane electrodes were prepared by doping rare earth element Ce into manganese oxide (MnOx) via sol–gel method, and coated on the porous Ti substrate membrane electrode. In order to evaluate electrocatalytic activity of the Ce–MnOx/Ti electrode, a functional electrocatalytic membrane reactor (ECMR), which assembled a Ce–MnOx/Ti electrode as an anode and a stainless steel mesh as a cathode, has been employed for phenolic wastewater treatment. The results revealed that a new crystal form Ce2O3 appeared after doping Ce into the system, and the urchinlike morphology and smaller grain size of Ce–MnOx led the catalytic activity of the composite membrane electrode to improve. Moreover, the 25 mol% Ce–MnOx/Ti (C25MT) composite membrane electrode represented the best activity in the degradation of phenolic wastewater. During ECMR with C25MT operation under the conditions of 450 mg/L phenolic wastewater (500 mL), residence time of 5 min, pH of 1–13 and current density of 0.3–1.2 mA/cm2, the highest degradation efficiency of phenolic wastewater achieved in current density of 0.9 mA/cm2 and pH of 7, that is, the achieved remove rate of phenol, COD and TOC were 99.61, 93.12, and 84.23%, respectively. Finally, the effective reusability of C25MT composite membrane electrode was assessed, and a proposed reaction mechanism has been analyzed in the ECMR operation.












Similar content being viewed by others
References
Gestel TV, Hauler F, Bram M, Meulenberg WA, Buchkremer HP (2014) Synthesis and characterization of hydrogen-selective sol–gel SiO2 membranes supported on ceramic and stainless steel supports. Sep Purif Technol 121(2):20–29
Wang H, Wang H, Li J, Bin D, Yin Z, Kang J, He B (2014) An electrocatalytic reactor for the high selectivity production of sodium 2,2,3,3-tetrafluoropropionate from 2,2,3,3-tetrafluoro-1- propanol. Electrochim Acta 123:33–41
Lin Y, Zou D, Chen X, Qiu M, Kameyama H, Fan Y (2015) Low temperature sintering preparation of high-permeability TiO2/Ti composite membrane via facile coating method. Appl Surf Sci 349:8–16
Rodrigues LA, Campos TMB, Alvarez-Mendes MO, Coutinho ADR, Sakane KK, Thim GP (2012) Phenol removal from aqueous solution by carbon xerogel. J Sol–Gel Sci Technol 63(2):202–210
Jiang SP, Chan SH (2004) Development of Ni/Y2O3-ZrO2 cermet anodes for solid oxide fuel cells. Mater Sci Technol 20(20):1109–1118
Yin Y, Li X, Shu Y, Guo X, Bao H, Li W, Zhu Y, Li Y, Huang X (2017) Fabrication of electrophoretically deposited, self-assembled three-dimensional porous Al/CuO nanothermite films for highly enhanced energy output. Mater Chem Phys 194:182–187
Ji Y, Liu J, Liu X, Yuen MMF, Fu XZ, Yang Y, Sun R, Wong CP (2017) 3D porous Cu@Cu2O films supported Pd nanoparticles for glucose electrocatalytic oxidation. Electrochim Acta 248:299–306
Fang X, Yin Z, Wang H, Li J, Liang X, Kang J, He B (2015) Controllable oxidation of cyclohexane to cyclohexanol and cyclohexanone by a nano-MnOx/Ti electrocatalytic membrane reactor. J Catal 329:187–194
ERARSLAN Y (2013) Wear performance of in-situ aluminum matrix composite after micro-arc oxidation. Trans Nonferrous Metal Soc 23(2):347–352
Chen F, Yu P, Zhang Y (2017) Healing effects of LDHs nanoplatelets on MAO ceramic layer of aluminum alloy. J Alloy Compd 711:342–348
Xue W, Wu X, Li X, Tian H (2006) Anti-corrosion film on 2024/SiC aluminum matrix composite fabricated by microarc oxidation in silicate electrolyte. J Alloy Compd 425(1):302–306
Xu L, Li M, Xu W (2015) Preparation and characterization of Ti/SnO2-Sb electrode with copper nanorods for AR 73 removal. Electrochim Acta 166:64–72
Buddee S, Wongnawa S (2015) Removal of dyes by photocatalytically active curcumin-sensitized amorphous TiO2 under visible light irradiation. J Sol–Gel Sci Technol 75(1):152–163
Zhang Q, Cheng X, Zheng C, Feng X, Qiu G, Tan W, Liu F (2011) Roles of manganese oxides in degradation of phenol under UV–Vis irradiation: adsorption, oxidation, and photocatalysis. J Environ Sci 23(11):1904–1910
Bose VC, Biju V (2015) Structure, cation valence states and electrochemical properties of nanostructured Mn3O4. Mater Sci Semicond Process 35:1–9
El-Deab MS, Ohsaka T (2007) Electrocatalysis by design: effect of the loading level of Au nanoparticles-MnOx nanoparticles binary catalysts on the electrochemical reduction of molecular oxygen. Electrochim Acta 52(5):2166–2174
Massa A, Hernández S, Lamberti A, Galletti C, Russo N, Fino D (2017) Electro-oxidation of phenol over electrodeposited MnOx nanostructures and the role of a TiO2 nanotubes interlayer. Appl Catal B Environ 203:270–281
Sotgiu G, Foderà M, Marra F, Petrucci E (2015) Production and characterization of manganese oxide-based electrodes for anodic oxidation of organic compounds. Chem Eng Trans 41:115–120
Wu X, Si Z, Li G, Weng D, Ma Z (2011) Effects of cerium and vanadium on the activity and selectivity of MnOx-TiO catalyst for low-temperature NH-SCR. J Rare Earth 29(1):64–68
Ching S, Carmichael JR, Eichelberger LA, Kriz DA, Alley KA, Jackvony SE (2016) Influence of Fe, Cu, V, and Ce doping on morphology and catalytic activity of amorphous manganese oxide hollow spheres. Polyhedron 114:205–212
Yan S, Wang J, Zhong J, Chen Y, Liu Z, Cao H, Gong M (2008) Effect of metal doping into Ce0.52x/Ce0.5-0.223. J Rare Earth 26(6):841–845
Li X, Zhang S, Jia Y, Liu X, Zhong Q (2012) Selective catalytic oxidation of NO with O2 over Ce-doped MnOx/TiO2 catalysts. J Rare Earth 21(1):17–24
Xie A, Zhou X, Huang X, Ji L, Zhou W, Luo S, Yao C (2017) Cerium-loaded MnOx/attapulgite catalyst for the low-temperature NH3-selective catalytic reduction. J Ind Eng Chem 49:230–241
Li D, Tang J, Zhou X, Li J, Sun X, Shen J, Wang L, Han W (2016) Electrochemical degradation of pyridine by Ti/SnO2-Sb tubular porous electrode. Chemosphere 149:49
Zhang D, Wu J, Li B, Fan Y (2015) Preparation of ceramic membranes on porous Ti-Al alloy supports by an in-situ oxidation method. J Membr Sci 476(5):554–560
Xin Y (2012) Study on preparation and photocatalytic degradation of alachlor on TiO2/Ti photoelectrodes prepared by microarc oxidation method. Procedia Eng 27:538–545
Li J, Li J, Wang H, Cheng B, He B, Yan F, Yang Y, Guo W, Ngo HH (2013) ChemInform abstract: electrocatalytic oxidation of n‐propanol to produce propionic acid using an electrocatalytic membrane reactor. Chem Commun 44(37):4501–4503
Wang J, Liang X, Chen P, Di Z, Yang S, Liu Z (2016) Microstructure and photocatalytic properties of Ag/Ce4+/La3+ co-modified TiO2/Basalt fiber for ammonia–nitrogen removal from synthetic wastewater. J Sol–Gel Sci Technol 82(1):289–298
Wang H, Guan Q, Li J, Wang T (2014) Phenolic wastewater treatment by an electrocatalytic membrane reactor. Catal Today 236:121–126
Bekermann D, Barreca D, Gasparotto A, Maccato C (2013) ChemInform abstract: multi-component oxide nanosystems by chemical vapor deposition and related routes: challenges and perspectives. Cheminform 44(14):6347–6358
Chang IS, Clech PL, Jefferson B, Judd S (2002) Membrane fouling in membrane bioreactors for wastewater treatment. J Environ Eng 128(11):1018–1029
Yu T, Meng L, Zhao QB, Shi Y, Hu HY, Lu Y (2017) Effects of chemical cleaning on RO membrane inorganic, organic and microbial foulant removal in a full-scale plant for municipal wastewater reclamation. Water Res 113:1
Yang Y, Li J, Wang H, Song X, Wang T, He B, Liang X, Ngo HH (2011) An electrocatalytic membrane reactor with self-cleaning function for industrial wastewater treatment. Angew Chem Int Ed Engl 50(9):2148–2150
Yang Y, Wang H, Li J, He B, Wang T, Liao S (2012) Novel functionalized nano-TiO2 loading electrocatalytic membrane for oily wastewater treatment. Environ Sci Technol 46(12):6815–6821
Guan Q, Wang H, Li J, Li X, Yang Y, Wang T (2013) Optimization of an electrocatalytic membrane reactor for phenolic wastewater treatment by response surface methodology. J Water. Sustainability 3(1):17–28
Fang X, Yin Z, Wang H, Li J, Liang X, Kang J, He B (2015) Controllable oxidation of cyclohexane to cyclohexanol and cyclohexanone by a nano-MnOx/Ti electrocatalytic membrane reactor. J Catal 329:187–194
Weisser MA, Van Petegem S, Cervellino A, Van Swygenhoven H (2015) On the origin of cementite diffraction peak broadening during tensile deformation at ambient temperatures. Int J Plast 66:138–144
Ettireddy PR, Ettireddy N, Mamedov S, Boolchand P, Smirniotis PG (2007) Surface characterization studies of TiO2 supported manganese oxide catalysts for low temperature SCR of NO with NH3. Appl Catal B Environ 76(1–2):123–134
Zhou GW, Kang YS (2004) Synthesis and structural properties of manganese titanate MnTiO3 nanoparticle. Mater Sci Eng C 24(1–2):71–74
Li M, Liu Z, Hu Y, Wang M, Li H (2008) Effect of doping elements on catalytic performance of CeO2-ZrO2 solid solutions. J Rare Earth 26(3):357–361
Etemadi B, Mazloom J, Ghodsi FE (2017) Phase transition and surface morphology effects on optical, electrical and lithiation/delithiation behavior of nanostructured Ce-doped V2O5 thin films. Mater Sci Semicond Process 61:99–106
Xu AW, Gao Y, Liu HQ (2002) The preparation, characterization, and their photocatalytic activities of rare-earth-doped TiO2 nanoparticles. J Catal 207(2):151–157
Khatun N, Rini EG, Shirage P, Rajput P, Jha SN, Sen S (2016) Effect of lattice distortion on bandgap decrement due to vanadium substitution in TiO2 nanoparticles. Mater Sci Semicond Process 50:7–13
Vashook V, Vasylechko L, Zosel J et al. (2004) Crystal structure and electrical conductivity of lanthanum–calcium chromites–titanates La1-xCaxCr1-yTiyO3-d (x = 0–1, y = 0–1). J Solid State Chem 177:3784–3794
Duan L et al. (2013) J Phys Conf Ser 418 012129
Mohite RG, Garg A (2017) Performance of heterogeneous catalytic wet oxidation for the removal of phenolic compounds: catalyst characterization and effect of pH, temperature, metal leaching and non-oxidative hydrothermal reaction. J Environ Chem Eng 5(1):468–478
Liu H, Vecitis CD (2012) Reactive transport mechanism for organic oxidation during electrochemical filtration: mass-transfer, physical adsorption, and electrontransfer. J Phys Chem C 116(1):374–383
Yang W, Yang S, Sun W, Sun G, Xin Q (2006) Nanostructured palladium-silver coated nickel foam cathode for magnesium-hydrogen peroxide fuel cells. Electrochim Acta 52(1):9–14
Shi ZL, Du C, Yao SH (2011) Preparation and photocatalytic activity of cerium doped anatase titanium dioxide coated magnetite composite. Photochem Photobiol 42(4):652–657
Wei X, Wang H, Yin Z, Qaseem S, Li J (2017) Tubular electrocatalytic membrane reactor for alcohol oxidation: CFD simulation and experiment. Chin J Chem Eng 25(1):18–25
Samet Y, Agengui L, Abdelhédi R (2010) Electrochemical degradation of chlorpyrifos pesticide in aqueous solutions by anodic oxidation at boron-doped diamond electrodes. Chem Eng J 161(1–2):167–172
Li J, Li J, Wang H, Cheng B, He B, Yan F, Yang Y, Guo W, Ngo HH (2013) ChemInform abstract: electrocatalytic oxidation of n‐propanol to produce propionic acid using an electrocatalytic membrane reactor. Chem Commun 49(40):4501–4503
Han YF, Chen L, Ramesh K, Widjaja E, Chilukoti S, Surjami IK, Chen J (2008) Kinetic and spectroscopic study of methane combustion over α -Mn2O3 nanocrystal catalysts. J Catal 253(2):261–268
Shi ZL, Du C, Yao SH (2011) Preparation and photocatalytic activity of cerium doped anatase titanium dioxide coated magnetite composite. J Taiwan Inst Chem Eng 42(4):652–657
Xie Y, Yuan C (2003) Visible-light responsive cerium ion modified titania sol and nanocrystallites for X-3B dye photodegradation. Appl Catal B Environ 46(2):251–259
Xue W, Zhang G, Xu X, Yang X, Liu C, Xu Y (2011) Preparation of titania nanotubes doped with cerium and their photocatalytic activity for glyphosate. Chem Eng J 167(1):397–402
Zhou L, Zhang J, He J, Hu Y, Tian H (2011) Control over the morphology and structure of manganese oxide by tuning reaction conditions and catalytic performance for formaldehyde oxidation. Mater Res Bull 46(10):1714–1722
Jiang S, Zhang H, Yan Y (2015) Catalytic wet peroxide oxidation of phenol wastewater over a novel Cu–ZSM-5 membrane catalyst. Catal Commun 71:28–31
Acknowledgements
The authors gratefully acknowledge the financial support by National Natural Science Foundation of China (21676200), the Program for Innovative Research Team in University of Ministry of Education of China (Grand no. IRT-17R80), National key research and development plan (2016YFC0400506).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Highlights
-
An electrocatalytic membrane reactor (ECMR) was assembled with Ce-MnOx/Ti as anode.
-
The electrocatalytic activity of ECMR was evaluated through phenol degradation.
-
The effect pH and current density on the electrocatalytic activity of ECMR was studied.
-
ECMR with 25 mol% Ce-MnOx/Ti exhibited the highest electrocatalytic performance.
Rights and permissions
About this article
Cite this article
Zhang, D., Liang, X., Yang, S. et al. Investigation of electrocatalytic activity of nanostructure Ce-doped MnO x sol–gel coating deposited on porous Ti membrane electrode. J Sol-Gel Sci Technol 86, 468–478 (2018). https://doi.org/10.1007/s10971-018-4620-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-018-4620-3