Abstract
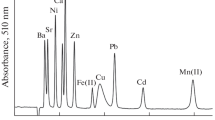
Systematic studies of silica gels with covalently immobilized thiosemicarbazide and formazan groups under the conditions of competitive sorption from multicomponent systems were conducted. A methodological approach to determine the selectivity of the modified sorption material with regard to Cu(II), Ni(II), Co(II), Cd(II), and Zn(II) was proposed. Solid-phase extraction in equilibrium conditions of Cu(II), Zn(II), Co(II), Cd(II), and Ni(II) on a silica gel with covalently immobilized thiosemicarbazide and formazan groups in the conditions of competitive sorption was studied. The possibility to use the pseudo-second-order kinetic equation for assessment of mutual influence at competitive sorption has been shown. We found that sorption from multicomponent solutions proceeds as a non-additive process under the conditions of an excess of functional groups.






Similar content being viewed by others
References
Pujari SP, Scheres L, Marcelis AT, Zuilhof H (2014) Covalent surface modification of oxide surfaces. Angew Chem Int Ed Engl 53:2–36. https://doi.org/10.1002/anie.201306709
Terada K (1991) Preconcentration of trace elements by sorption. Anal Sci 7:187–198. https://doi.org/10.2116/analsci.7.187
Biernat Jan F, Konieczka P (1994) Complexing and chelating agents immobilized on silica gel and related materials and their application for sorption of inorganic species. Sep Purif Methods 23:77–348. https://doi.org/10.1080/03602549408006624
Garg BS, Sharma RK, Bhojak N, Mittal S (1999) Chelating resins and their application in the analysis of trace metal ions. Microchem J 61:94–114. https://doi.org/10.1006/mchj.1998.1681
Price Peter M, James HC, Duncan JM (2000) Modified silicas for clean technology. J Chem Soc Dalton Trans: 101–110. https://doi.org/10.1039/A905457J
Voronkov MG, Vlasova NN, Pozhidaev YuN (2000) Organosilicon ion-exchange and complexing adsorbents. Appl Organomet Chem 14:287–303. https://doi.org/10.1002/pat.743
Sharma RK, Mittal S, Koe M (2003) Analysis of trace amounts of metal ions using silica-based chelating resins: a green analytical method. Crit Rev Anal Chem 33:183–197. https://doi.org/10.1080/713609163
Jal PK, Patel S, Mishra BK (2004) Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta 62:1005–1028. https://doi.org/10.1016/j.talanta.2003.10.028
Zougagh M, Cano Pavon JM, Garcia de Torres A (2005) Chelating sorbents based on silica gel and their application in atomic spectrometry. Anal Bioanal Chem 381:1103–1113. https://doi.org/10.1007/s00216-004-3022-2
Kara D, Fisher A (2012) Modified silica gels and their use for the preconcentration of trace elements. Sep Purif Rev 41:267–317. https://doi.org/10.1080/15422119.2011.608765
Sierra I, Perez-Quintanilla Dn (2013) Heavy metal complexation on hybrid mesoporous silicas: an approach to analytical applications. Chem Soc Rev 42:3792–3807. https://doi.org/10.1039/C2CS35221D
Ding C, Cheng W, Wang X, Wu ZY, Sun Y, Chen C, Wang X, Yu SH (2016) Competitive sorption of Pb(II), Cu(II) and Ni(II) on carbonaceous nanofibers: a spectroscopic and modeling approach. J Hazard Mater 313:253–261. https://doi.org/10.1016/j.jhazmat.2016.04.002
Selim HM, Zhang H (2013) Modeling approaches of competitive sorption and transport of trace metals and metalloids in soils: a review. J Environ Qual 42(3):640–653. https://doi.org/10.2134/jeq2012.0323
Shaheen SM, Tsadilas CD, Rinklebe J (2013) A review of the distribution coefficients of trace elements in soils: influence of sorption system, element characteristics, and soil colloidal properties. Adv Colloid Interface Sci 201-202:43–56. https://doi.org/10.1016/j.cis.2013.10.005
Covelo EF, Vega FA, Andrade ML (2006) Competitive sorption and desorption of heavy metals by individual soil components. J Hazard Mater 140(1-2):308–315. https://doi.org/10.1016/j.jhazmat.2006.09.018
Xiao B, Thomas KM (2004) Competitive adsorption of aqueous metal ions on an oxidized nanoporous activated carbon. Langmuir 20(11):4566–4578. https://doi.org/10.1021/la049712j
Cheng W, Ding C, Wang X, Wu Z, Sun Y, Yu S, Hayat T, Wang X (2016) Competitive sorption of As(V) and Cr(VI) on carbonaceous nanofibers. Chem Eng J 293:311–318. https://doi.org/10.1016/j.cej.2016.02.073
Konshina DN, Furina (Danilova) AV, Temerdashev ZA, Gurinov AA, Konshin VV (2014) Immobilization of guanazyl functional groups on silica for solid-phase extraction of metal ions. Anal Lett 47:2665–2681. https://doi.org/10.1080/00032719.2014.917421
Konshina DN, Open’ko VV, Temerdashev ZA, Gurinov AA, Konshin VV (2016) Synthesis of novel silicagel-supported thiosemicarbazide and its properties for solid phase extraction of mercury. Sep Sci Technol 51:1103–1111. https://doi.org/10.1080/01496395.2016.1143005
Konshina DN, Danilova AV, Temerdashev ZA, Bolotin SN, Gurinov AA, Konshin VV (2016) Preparation and properties of silica gel with immobilized formazan group. Russ J Appl Chem 89:476–483. https://doi.org/10.1134/S107042721604011X
Sergio P (2006) A note on Fisher’s correlation coefficient. Appl Math Lett 19:499–502. https://doi.org/10.1016/0021-9797(74)90252-5
Filip E, Nadejde C, Creanga D, Dorohoi D (2009) Spectral investigation of triphenylformazan derivatives in ultra violet light. Rom J Phys 54:649–657
João P, Vareda LD (2017) Functionalized silica xerogels for dsorption of heavy metals from groundwater and soils. J Sol Gel Sci Technol 84:400–408. https://doi.org/10.1007/s10971-017-4326-y
Giles CH, Smith D, Huitson A (1974) A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J Colloid Interface Sci 47:755–765. https://doi.org/10.1016/0021-9797(74)90252-5
Ho YS, McKay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34:735–742. https://doi.org/10.1016/S0043-1354(99)00232-8
Fu L, Zhang L, Wang S, Zhang G, Peng J (2017) Selective recovery of Au(III) from aqueous solutions by nano-silica grafted with 4-(aminomethyl) pyridine. J Sol Gel Sci Technol 83:467–477
Acknowledgements
This publication was financially supported by the Russian Ministry of Education and Science (project no. 4.4892.2017/8.9). This work was accomplished with the use of the scientific equipment of the Centre of Collective Employment “Ecoanalytical Centre”, Kuban State University (RFMEFI59317Х0008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Highlights
-
Sorption of Cu (II), Zinc (II), Ni(II), Co(II), Cd (II) from multicomponent solutions proceeds as a non-additive process under the conditions of an excess of functional groups
-
Copper (II) and Zinc (II) have shown selectivity at extraction on the modified silica gels
-
The possibility to use the pseudo-second order kinetic equation for assessment of mutual influence at competitive sorption has been shown
Rights and permissions
About this article
Cite this article
Konshina, D.N., Temerdashev, Z.A. & Konshin, V.V. Some aspects of estimation of extraction selectivity under the conditions of competitive sorption on modified silica gels. J Sol-Gel Sci Technol 86, 34–41 (2018). https://doi.org/10.1007/s10971-018-4619-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-018-4619-9