Abstract
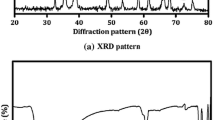
Gamma-ray irradiation assisted polyacrylamide gel route was used to prepare CoAl2O4 nanoblue pigments. In this route, citric acid was used as a carboxyl and hydroxyl type chelating agent. The phase purity, morphology, and optical and fluorescence properties of as-prepared samples were analyzed via X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectrometer, transmission electron microscopy (TEM), UV–Visible spectrophotometer, and a confocal Raman system. XRD analysis indicates that the xerogel sintered at relatively low temperature (500 °C) to obtain single phase CoAl2O4 nanopowders. The primary crystal of CoAl2O4 nanoblue pigment is only 28 nm characterized by TEM, which is more likely to be realized with high uniformity than that the CoAl2O4 nanopowders prepared by conventional polyacrylamide gel route. Optical properties of CoAl2O4 nanoblue pigment shows that the optical energy gap (Eg) of nanoblue pigment increases with the decrease of crystallite size. The CIE parameter of CoAl2O4 nanoblue pigment indicates that a different sintering temperature causes a variation in the color of nanoparticles. The fluorescence spectra show that a major blue emission band around 400 nm and a weaker side band located at 430 nm are observed when the excitation wavelength is 325 nm. The chelation mechanism and fluorescence mechanism of the CoAl2O4 nanoblue pigment have been analyzed based on the experimental results.

A cubic nano-CoAl2O4 pigments were synthesized by γ-ray irradiation assisted polyacrylamide gel route. The nano-CoAl2O4 pigments with high uniformity than that the CoAl2O4 nanopowders prepared by conventional polyacrylamide gel route. The SAED pattern revealed that the CoAl2O4 nanoparticles possess interplanar spacing of 2.8651, 2.4434, 2.0258, 1.5586, 1.4327, 1.3657, and 1.1549 Å corresponding to the (220), (311), (400), (511), (440), (531), and (444) planes, respectively. The UV–Vis absorption spectrum shows three obvious absorption peaks at 551, 590, and 628 nm and the CIE parameter is consistent with the real photos.









Similar content being viewed by others
References
Jafari M, Hassanzadeh-Tabrizi SA, Ghashang M, Pournajaf R (2014) Ceram Int 40:11877–11881
Bulte JWM, Kraitchman DL (2004) NMR Biomed 17:484–499
Zayat M, Levy D (2000) Chem Mater 12:2763–2769
Son SJ, Reichel J, He B, Schuchman M, Lee SB (2005) J Am Chem Soc 127:7316–7317
Basak J, Hardia N, Saxena S, Dixit R, Dwivedi R, Bhadauria S, Prasad R (2007) Ind Eng Chem Res 46:7039–7044
de Herval LKS, Tuncer Arslanlar Y, Ayvacikli M, Iikawa F, Nobrega JA, Pizani PS, Galvão Gobato Y, Can N, Henini M, de Godoy MPF (2015) J Lumin 163:17–20
Yoon S, Bierwagen J, Trottmann M, Walfort B, Gartmann N, Weidenkaff A, Hagemann H, Pokrant S (2015) J Lumin 167:126–131
Ji L, Tang S, Zeng HC, Lin J, Tan KL (2001) Appl Catal A 207:247–255
Melo DMA, Cunha JD, Fernandes GDG, Bernardi MI, Melo MAF, Martinelli AE (2003) Mater Res Bull 38:1559–1564
Li W, Li J, Guo J (2003) J Eur Ceram Soc 23:2289–2295
Otero Area´n CO, Mentruit MP, Platero EE, Xamena FXL, Parra JB (1999) Mater Lett 39:22–27
Merikhi J, Jungk HO, Feldmann CJ (2000) J Mater Chem 10:1311–1314
Stangar UL, Orel B, Krajnc M (2003) J Sol Gel Sci Technol 26:771–775
Manikandan A, Durka M, Amutha Selvi M, Arul Antony S (2016) J Nanosci Nanotechno 16:448–456
Gama L, Ribeiro MA, Barros BS, Kiminami RHA, Weber IT, Costa ACFM (2009) J Alloy Comp 483:453–455
Gholami T, Salavati-Niasari M, Varshoy S (2016) J Hydrog Energ 41:9418–9426
Ahmed IS (2011) Mater Res Bull 46:2548–2553
Torkian L, Daghighi M (2014) Adv Powder Technol 25:739–744
Chandradass J, Balasubramanian M, Kim KH (2010) J Alloy Comp 506:395–399
Duan XL, Pan M, Yu FP, Yuan DR (2011) J Alloy Comp 509:1079–1083
Kurajica S, Popovic J, Tkalcec E, Grzeta B, Mandic V (2012) Mater Chem Phys 135:587–593
Xi XL, Nie ZR, Ma LW, Li L, Xu XY, Zuo TY (2012) Powder Technol 226:114–116
Aly KA, Khalil NM, Algamal Y, Saleem QMA (2016) J Alloy Comp 676:606–612
Peymannia M, Soleimani-Gorgani A, Ghahari M, Jalili M (2015) Ceram Int 41:9115–9121
Peymannia M, Soleimani-Gorgani A, Ghahari M, Najafi F (2014) J Eur Ceram Soc 34:3119–3126
Wang QK, Chang QB, Wang YQ, Wang X, Zhou JE (2016) Mater Lett 173:64–67
Chen ZZ, Shi EW, Li WJ, Zheng YQ, Zhong WZ (2002) Mater Lett 55:281–284
Jafari M, Hassanzadeh-Tabrizi SA (2014) Powder Technol 266:236–239
Zhao X, Yang H, Cui Z, Li R, Feng W (2017) Mater Technol 32:870–880
Saket-Oskoui M, Khatamian M, Nofouzi K, Yavari A (2014) Adv Powder Technol 25:1634–1642
Di LJ, Yang H, Xian T, Chen XJ (2017) Materials 10:1118
Zheng CX, Yang H, Cui ZM, Zhang HM, Wang XX (2017) Nanoscale Res Lett 12:608
Wang SF, Lv HB, Zhou XS, Fu YQ, Zu XT (2014) Nanosci Nanotech Lett 6:758–771
Sin A, Odier P (2000) Adv Mater 12:649–652
Wang SF, Li DM, Yang CQ, Sun GA, Zhang J, Xia YH, Xie CM, Yang GX, Zhou M, Liu W (2017) J Sol Gel Sci Technol 84:169–179
Wang F, Yang H, Zhang YC (2018) Mat Sci Semicon Proc 73:58–66
Gabrovska M, Crisan D, Stanica N, Nikolova D, Bilyarska L, Crisan M, Edreva-Kardjieva R (2014) Rev Roum Chim 59:445–450
Renouprez AJ (1992) Acta Phys Pol Α 82:295–308
Ianos R, Borcǎnescu S, Lazaǎu R (2014) Chem Eng J 240:260–263
Salavati-Niasari M, Farhadi-Khouzani M, Davar F (2009) J Sol Gel Sci Technol 52:321–327
Mindru I, Marinescu G, Gingasu D, Patron L, Ghica C, Giurginca M (2010) Mater Chem Phys 122:491–497
Ahmed IS, Dessouki HA, Ali AA (2008) Spectrochim Acta A 71:616–620
de Souza LKC, Zamian JR, daRocha Filho GN, Soledade LEB, dos Santos IMG, Souza AG, Scheller T, Angelica RS, da Costa CEF (2009) Dyes Pigments 81:187–192
El Habra N, Crociani L, Sada C, Zanella P, Casarin M, Rossetto G, Carta G, Paolucci G (2007) Chem Mater 19:3381–3386
Rangappa D, Naka T, Kondo A, Ishii M, Kobayashi T, Adschiri T (2007) J Am Chem Soc 129:11061–11066
Agilandeswari K, Ruban Kumar A (2015) AIP Conf Proc 1665:120022-1–120022-3. https://doi.org/10.1063/1.4918129
Ahmad F (2014) J Alloy Comp 586:605–610
Zheng WC, Wu XX, Fang W, Mei Y (2007) Spectrochim Acta A 66:1295–1299
Ho C, Yu JC, Kwong T, Mak AC, Lai S (2005) Chem Mater 17:4514–4522
Jayasree S, Manikandan A, Mohideen AM, Barathiraja C, Antony SA (2015) Adv Sci Eng Med 7:672–682
Pelagotti A, Pezzati L, Bevilacqua N, Vascotto V, Reillon V, Daffara C (2005) A study of UV fluorescence emission of painting materials. ‘05–8th International Conference on Non-Destructive Investigations and Microanalysis for the Diagnostics and Conservation of the Cultural and Environmental Heritage. Lecce, CD-ROM, A97, 2005
Alves E, Marques C, da Silva RC, Monteiro T, Soares J, McHargue C, Ononye LC, Allard LF (2003) Nucl Instrum Meth B 207:55–62
Monteiro T, Soares MJ, Santos L, Boemare C, Cascalheira J, Alves LC, Alves E (2002) Radiat Eff Defect S 157:1117–1122
Kuleshov NNV, Mikhailov VP, Scherbitsky VG, Prokoshin PV, Yumashev KV (1993) J Lumin 55:265–269
Ahn KS, Yan YF, Kang MS, Kim JY, Shet S, Wang HL, Turner J, Al-Jassim M (2009) Appl Phys Lett 95:022116
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51662027, and 61540043).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Highlights
-
CoAl2O4 nanoblue pigments were prepared by γ-ray irradiation assisted polyacrylamide gel route.
-
CoAl2O4 nanoblue pigments with high uniformity.
-
Sintering temperature causes a variation in the color and Eg value of CoAl2O4 nanoparticles.
-
The chelation and fluorescence mechanisms of the CoAl2O4 nanoblue pigment have been analyzed.
Rights and permissions
About this article
Cite this article
Gao, H., Yang, H., Wang, S. et al. A new route for the preparation of CoAl2O4 nanoblue pigments with high uniformity and its optical properties. J Sol-Gel Sci Technol 86, 206–216 (2018). https://doi.org/10.1007/s10971-018-4609-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-018-4609-y