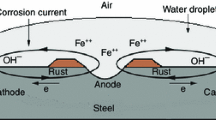
Abstract
Dibutil-dilaurate tin was proposed as a neutral poly-condensation catalyst for the formation of organically modified ceramics between the TEOS and poly-di-methyl-di-siloxane, which was named organic modified silane-dibutil-dilaurate tin. The properties of this material were comparison with an organic modified silane synthetized under similar condition but with acid catalyst. Infrared spectroscopy and NMR 29Si indicated the chemical bonding between the polymer and the silica fragments formed by the TEOS, when dibutil-dilaurate tin was used as catalyst. Also, organic modified silanes were applied on an aluminum surface (Al-6061) as an anticorrosive coating; SEM showed that organic modified silane-dibutil-dilaurate tin coating was deposited homogeneously on the metal substrate; while organic modified silane-acid form a heterogeneous film. Anticorrosive behavior was assessed by gravimetric corrosive tests and it was compared with a commercial polymer film. The results showed an excessive wear on the metal surface by means of intra-grain corrosion; the corrosion rate was determined as: organic modified silane-dibutil-dilaurate tin < organic modified silane-acid< polymer coating < aluminum surface.
Graphical Abstract






Similar content being viewed by others
References
Brinker J, Scherer GW (1990) Sol-gel science: the physics and chemistry of sol-gel processing. Academic Press INC, San Diego
Schmidt H, Jonschker G, Goedicke S, Menning M (2000) J Sol Gel Sci Technol 19:39–51
Singh LP, Bhattacharyya SK, Kumar R, Mishra G, Sharma U, Singh G, Ahalawat S (2014) Adv Colloid Interface Sci 214: 17–37
Méndez-Vivar J (2006) J Sol Gel Sci Technol 38 (2):159–164
Salazar-Hernández C, Zárraga R, Alonso S, Sugita S, Calixto S, Cervantes J, (2009) J Sol Gel Sci Technol 49(3):301–310
Salazar-Hernández C, Puy Alquiza MJ, Salgado P, Cervantes J (2010) Appl Organomet Chem 24(6):481–488
Mangun CL, Mader AC, Sottos NR, White SR. (2010) Polymer 51:4063–4068
Munteanu D (1997) Moisture cross-linkable silane-modified polyolefins In: Al-Malaika S (ed) Reactive modifiers for polymer. Springer, pp 196–265
Salazar-Hernández C, Alonso S, Cervantes J (2010) J Sol Gel Sci Technol 54(1):77–82
Boyer IJ (1989) Toxicology 55:253–298
Rudel H (2003) Ecotoxicol Environ Saf 56:180–189
Cervantes J, Zarraga R, Salzar-Hernández C (2012) Appl Organometall Chem 26:157–163
Minami T (2013) J Sol Gel Sci Technol 65:4–11
Alongi J, Malucelli G (2012) J Mater Chem 22:21805–21809
Zheludkevich ML, Salvado IM, Ferreira MGS (2005) J Mater Chem 15:5099–5111
Roussi E, Tsetsekou A, Skarmoutsou A, Charitidis CA, Karantonis A (2013) Surf Coat Technol 232:131–141
Kulinichn SA, Akhtar AS (2012) Russ J Non Ferr Met 53(2):176–203
Whelan M, Cassidy J, Duffy B (2013) Surf Coat Technol 235:86–96
Zhou CH, Lu X, Xin A, Liu J (2013) Corros Sci 70:145–151
Pathak SS, Khanna AS, Sinha TJM (2006) Corros Rev 24 (5–6):281–306
Galio AF, Lamaka SV, Zheludkevich ML, Dick LFP, Muller IL, Ferreira MGS (2010) Surf Coat Technol 204:1479–1486
Scherer GW (1992) J Non Cryst Solids 144:210–216
Woods ME, Vreugdenhil (2006) J Mater Sci 41:7545–7554
Gobara M, Kamel H, Akid R, Baraka A (2015) Prog Org Coat 89:57–66
Eduok U, Suleiman R, Khaled M, Akid R (2016) Prog Org Coat 93:97–108
Khramov AN, Voevodin NN, Balbyshev VN, Donley MS (2004) Thin Solid Film 447–448:549–557
Liu X, Xu Y, Ben K, Chen Z, Wang Y, Guan Z (2015) Appl Surf Sci 339:94–101
Lin HT, Bescher E, Mackenzie JD, Dai H, Stafsudd OM (1992) J Mater Sci 27:5523–5528
Tiwari I, Singh KP, (2012) Russ J Gen Chem 82(1):157–167
Ho WJ, Yuan CHJ, Reiko O (2006) Anal Chim Acta 572:248–252
Chakrabarti EOK, Jung HY, Whang CM (2002) Mater Sci Eng B 90:60–66
Mackenzie JD, Huang Q, Iwamoto T, (1996) J Sol Gel Sci Technol 7:151–161
Battisha IK, Beyally AE, Mongy SE, Nahrawi AM (2007) J Sol Gel Sci Technol 41:129–137
Launer PJ (1987) Infrared analysis of organosilicon compounds: spectra structure correlation silicon compounds. In: Arkles B (ed) Gelest Inc
Marsmann HC (199) 29Si NMR in molecular sciences and chemical engineering from encyclopedia of spectroscopy and apectrometry. 2nd edn., pp 2539–2549
McCafferty E (2010) Getting started on the basic. In: Corrosion science. Springer, London pp 24–25
Acknowledgements
The authors wish to acknowledge the financial support of the CONACyT through grant CB-186327/2012 register as SIP-2013-Re/099, as well as to Ernan Hernández Cantero for her technical support in this work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hernández, C.S., Hernández, M.S., Cerritos, R.C. et al. DBTL as neutral catalyst on TEOS/PDMS anticorrosive coating. J Sol-Gel Sci Technol 81, 405–412 (2017). https://doi.org/10.1007/s10971-016-4198-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-4198-6