Abstract
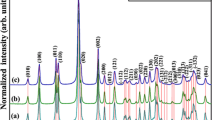
Dy3+/Yb3+ co-doped Gd2O3 monodispersed nanocrystals have been synthesized using the sodium dodecyl benzene sulfonate (SDBS) as the surfactant. The SDBS surfactant plays a critical role in the forming of monodisperse Gd2O3 structures with sphere-like morphologies. The growth mechanism of the nanocrystals was proposed in detail. The samples annealed at 400 °C for 4 h exhibit good crystallinity and narrow size distribution with average size of 6–9 nm. Green and red emission of Dy3+ related to 4G11/2–6H11/2 and 4G11/2–6H9/2 transition was observed under the laser excitation at 980 nm. An involvement of three photons has been observed for these emissions. The energy distribution of upconversion spectra depended on doping concentration and the intensity changed with the increasing of Dy3+ concentration. The mechanisms of upconversion by multi-photon absorption and energy transfer processes transitions were interpreted and explained.
Graphical Abstract









Similar content being viewed by others
References
Aebischer A, Heer S, Biner D et al (2005) Chem Phys Lett 407:124–128
Pollnau M, Gamelin DR, Lüthi SR, Güdel HU, Hehlen MP (2000) Phys Rev B 61:3337–3341
Suyver JF, Aebischer A, Biner D et al (2005) Opt Mater 27:1111–1130
Brigitte B, Belto D, Youping G, Alessandro C, Maurizio F (2014) Opt Eng 53:071814
Ahrén M, Selegård L, Klasson A (2010) Langmuir 26:5753
Hai G, Ning D, Min Y, Weiping Z, Liren L, Shangda XJ (2004) Phys Chem. B 108:19205–19209
Gan T, Zhanjun G, Xiaoxiao L et al (2011) J Phys Chem C 115:23790–23796
Jianle Z, Lifang L, Herman HYS et al (2007) Inorg Chem 46:5404–5410
Dwivedi Y, Rai SB (2009) Opt Mater 31:1472–1477
Lingling P, Bitao L, Tao H (2013) J. Rare Earths 31:650–654
Shixiu C, Tianmo L, Wen Z, Shahid H, Zhongchang W (2014) Nanosci Nanotechnol Lett 6:1087–1090
Shixiu C, Tianmo L, Wen Z, Shahid H, Zhongchang W (2014) Mater Lett 129:205–208
Aihua L, Qiang L, Zhiren Z, Liang S, Wenzhi W (2007) J Appl Phys 102:113102–113105
Duan Z, Zhang J, Hu L (2007) J Appl Phys 101:43110–43113
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (51302330), Science and Technology Research Foundation of the Education Commission of Chongqing City (KJ1501132), Chongqing Natural Science Foundation (cstc2015jcyjA50013), Talent Introduction Project of Chongqing University of Arts and Science (Nos. R2012CJ17, Y2013CJ25 and R2012CJ19) and new Material Development and Application Innovative Research Team of Higher Education in Chongqing of China (Grant No. 201042).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, L., Huang, M., Cao, S. et al. Enhanced upconversion in Dy3+, Yb3+ co-doped Gd2O3 monodisperse nanocrystals. J Sol-Gel Sci Technol 78, 307–312 (2016). https://doi.org/10.1007/s10971-015-3952-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3952-5