Abstract
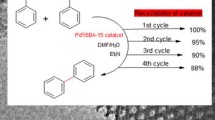
Here we describe a new platinum catalyst comprised of Pt(0) nanoparticles immobilized on a modified magnetic mesoporous silica support modified with electron donor groups (–N). The material is constituted of controlled pore size (2.4–4.1 nm) and serves as a template for the generation of Pt nanoparticles (2–4 nm). The catalytic activity of the supported Pt nanoparticles was investigated in the catalytic reduction of anthracene under ultra-mild conditions. A complete morphological characterization of the hybrid organic–inorganic composite which confirms the formation of the hybrid material is also given. The catalyst was easily recycled using a small magnet, and it could be reused at least twice without significant loss of its catalytic activity. ICP–OES reveals that after the recyclability study no leaching of Pt or Si could be detected in the products (<0.01 ppm) which confirms the chemical stability of the material allowing it to be used as a potential hydrogenation catalyst for solid–liquid reactions with facile catalyst recovery.
Graphical Abstract








Similar content being viewed by others
References
Atabaev TJ, Lee JH, Han DW, Hwang H, Hong NH (2013) Nanotechnology 24:345603–345610
Yin Y, Rioux RM, Erdonmez CK, Hughes S, Somorjai GA, Alivisatos AP (2004) Science 304:711–714
Jeong GH, Kim EG, Kim SB, Park ED, Kim SW (2011) Micropor Mesopor Mat 144:134–139
Carniato F, Bisio C, Paul G, Gatti G, Bertinetti L, Coluccia S, Marchese L (2010) J Mater Chem 20:5504–5509
Joo SH, Park JY, Tsung CK, Yamada Y, Yang P, Somorjai GA (2009) Nat Mater 8:126–131
Xie R, Wang H, Gao P, Xia L, Zhang Z, Zhao T, Sun Y (2015) Appl Catal A Gen 492:93–99
Malay O, Yilgor I, Menceloglu YZ (2013) J Sol–Gel Sci Technol 67:351–361
Jacinto MJ, Kiyohara PK, Masunaga SH, Jardim RF, Rossi LM (2008) Appl Catal A Gen 338:52–57
Fang Y, Chen Y, Li X, Zhou X, Li J, Tang W, Huang J, Jin J, Ma J (2014) J Mol Catal A Chem 392:16–21
Jacinto MJ, Santos OHCF, Landers R, Kiyohara PK, Rossi LM (2009) Appl Catal B Env 90:688–692
Murugan E, Jebaranjitham N (2015) Chem Eng J 259:266–276
Darwish MSA, Kunz U, Peuker UJ (2012) Appl Polym Sci 129:1806–1811
Qureshi ZS, Sarawade PB, Albert M, D’Elia V, Hedhili MN, Köhler K (2015) Chem Cat Chem 7:635–642
Foppa L, Dupont J, Scheeren C (2014) RSC 4:16583–16588
Lee DH, Jung JY, Jin MJ (2010) RSC 12:2024–2029
Ray S, Bhaumik A, Pramanik M, Mukhopadhyay C (2014) RSC 4:15441–15450
Carlier E, Guyot A, Revillon A (1992) Reac Polym 18:167–171
Rosenholm JM, Lindén M (2007) Chem Mater 19:5023–5034
Ortiz HIM, Mercado YP, Silva JAM, Maldonado YO, Castruita G, Cerda LAG (2013) Ceram Int 40:9701–9707
Tan SY, Ang CY, Li P, Yap QM, Zhao Y (2014) Chem Eur J 20:11276–11282
Niu D, Liu Z, Li Y, Luo X, Zhang J, Gong J, Shi J (2014) Adv Mater 26:4947–4953
Yang P, Gai S, Lin J (2012) Chem Soc Rev 41:3679–3698
Nador F, Moglie Y, Vitale C, Yus M, Alonso F, Radivoy G (2010) Tetrahedron 66:4318–4325
Liao W, Liu HW, Chen HJ, Chang WY, Chiu KH, Wai CM (2011) Chemosphere 82:573–580
Yuan T, Marshall WD (2005) J Hazard Mater B 126:149–157
Nelkenbaum E, Dror I, Berkowitz B (2007) Chemosphere 68:210–217
Li G, Liu Y, Du H (2015) Org Biomol Chem 13:2875–2878
Park J, An K, Hwang Y, Park JG, Noh HJ, Kim JY, Park JH, Hwang NM, Hyeon T (2004) Nat Mater 3:891
Kresge CT, Leonowics ME, Roth WJ, Vartuli JC, Beck JS (1992) Nature 359:710
Briggs D, Seah MP (1990) Practical surface analysis, vol 1 Auger and X-ray photoelectron spectroscopy. Willey, New York
Smith GC (1994) Surface analysis by electron spectroscopy. Plenum, New York
Scofield JH (1996) J Electron Spectrosc 8:129–132
Moulder JF, Stickle WF, Sobol PE, Bomben KD (1992) in: J. Chastian, Handbook of X-ray photoelectron spectroscopy, Perkin-Elmer Corp., Minnesota
Naumkin AV, Kraut-Vass A, Gaarenstroom SW, Powell CJ (2012)NIST X-ray photoelectron spectroscopy database, 20, V. 4.1. Retrieved from: http://srdata.nist.gov/xps/Version_his.aspx, last accessed: 04/03/2015 at 14:52
Acknowledgments
The authors are grateful to Fundação de Amparo a Pesquisa do Estado do Mato Grosso (FAPEMAT) and Conselho Nacional de Desenvolvimento Científico e Tecnológico(CNPq) for financial support, and indebted to LEFE (Brazil), LME-DEMA (Brazil) and LMC-UnB for the XPS, TEM and BET analyses, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacinto, M.J., Wizbiki, M., Justino, L.C. et al. Platinum-supported mesoporous silica of facile recovery as a catalyst for hydrogenation of polyaromatic hydrocarbons under ultra-mild conditions. J Sol-Gel Sci Technol 77, 298–305 (2016). https://doi.org/10.1007/s10971-015-3854-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3854-6