Abstract
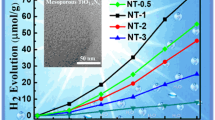
Mesoporous TiO2 hollow shells were synthesized by a conventional templating method which combines sol–gel coating and selective etching of the silica cores. Pt nanocatalysts were supported on these mesoporous TiO2 hollow shells varying the metal loading: 0, 1, 3, 5 and 7 % at calcination temperatures of 500 or 900 °C. The samples were characterized by transmission electron microscopy, X-ray diffraction, UV–Vis diffuse reflectance spectroscopy, and nitrogen physisorption. The mesostructures were observed by TEM and HRTEM to be smaller than 300 nm and the TiO2 shells had an average wall thickness of 40 nm. X-ray diffraction spectra revealed a pure anatase phase in samples calcined at 900 °C, whereas those calcined at 500 °C were amorphous. Under white light (UV and Visible) illumination, photocatalytic hydrogen production was measured from the samples suspended in an aqueous solution of methanol and compared to TiO2 (P25, Degussa) used as a reference. The highest hydrogen yields were achieved with the crystalline TiO2 hollow shells annealed at 900 °C containing 1 or 7 wt% Pt. The amorphous samples were observed to be inactive, at all metal loadings.
Graphical Abstract








Similar content being viewed by others
References
Lewis NS, Nocera DG (2006) Proc Natl Acad Sci USA 103:15729–15735
Armaroli N, Balzani V (2007) Angew Chem Int Ed 46:52–66
Acar C, Dincer I, Zamfirescu C (2014) Int J Energy Res 38:1903–1920
Kudo A, Miseki Y (2009) Chem Soc Rev 38:253–278
Yang J, Wang D, Han H, Li C (2013) Acc Chem Res 46:1900–1909
Jing D, Guo L, Zhao L, Zhang X, Liu H, Li M, Shen S, Liu G, Hu X, Zhang X, Zhang K, Ma L, Guo P (2010) Int J Hydrog Energy 35:7087–7097
Ashokkumar M (1998) Int J Hydrog Energy 23:427–438
Hagfeldt A, Graetzel M (1995) Chem Rev 95:48–499
Liu X, Li J, Zhang Y, Huang J (2015) J Chem Eur 21:7345–7349
Kochuveedu ST, Jang YH, Kim DH (2013) Chem Soc Rev 42:8467–8493
Patterson JD, Bailey BC (2007) Solid-state physics—introduction to the theory. Springer, Berlin
Reiss P, Protiere M, Li L (2009) Small 5:154–168
Joo JB, Lee I, Dahl M, Dae Moon G, Zaera F, Yin Y (2013) Adv Funct Mater 23:4246–4254
Joo JB, Zhang Q, Lee I, Dahl M, Zaera F, Yin Y (2012) Adv Funct Mater 22:166–174
Bian Z, Zhu J, Cao F, Lu Y, Li H (2009) Chem Commun 25:3789–3791
Wilson GJ, Matijasevich AS, Mitchell G, Schulz DR, Will JC (2006) Langmuir 22:2016–2027
Chen L, Zhao W, Jiao Y, He X, Wang J, Zhang Y (2007) Spectrochim Acta Part A 68:484–490
Yang J, Lee JY, Chen LX, Too H-P (2005) J Phys Chem B 109:5468–5472
Tauc J, Grigorovichi R, Vancu A (1966) Phys Status Solidi B 15:627–637
Lin W-C, Yang W-D, Huang I-L, Wu T-S, Chung Z-J (2009) Energy Fuels 23:2192–2196
Bacsa RR, Kiwi J (1998) Appl Catal B 16:19–29
Ohno T, Sarukawa K, Tokieda K, Matsumura M (2001) J Catal 203:82–86
Jakob M, Levanon H, Kamat PV (2003) Nano Lett 3:353–358
Subramanian V, Wolf EE, Kamat PV (2004) J Am Chem Soc 126:4943–4950
Burgeth G, Kisch H (2002) Coord Chem Rev 230:41–47
Wood A, Giersig M, Mulvaney P (2001) J Phys Chem B 105:8810–8815
Subramanian V, Wolf EE, Kamat PV (2003) J Phys Chem B 107:7479–7485
Cojocaru B, Neaţu Ş, Sacaliuc-Pârvulescu E, Lévy F, Pârvulescu VI, Garcia H (2011) Appl Catal B 107:140–149
Bumajdad A, Madkour M (2014) Phys Chem Chem Phys 16:7146–7158
Tian Y, Tatsuma T (2005) J Am Chem Soc 127:7632–7637
Furube A, Du L, Hara K, Katoh R, Tachiya M (2007) J Am Chem Soc 129:14852–14853
Sreethawong T, Laehsalee S, Chavadej S (2009) Catal Commun 10:538–543
Mankidy BD, Joseph B, Gupta VK (2013) Nanotechnology 24:405402
Acknowledgments
The authors acknowledge the financial supports of Consejo Nacional de Ciencia y Tecnología 166354, 251151, 153356, SIP 20150030 and SIP 20150621. FPH is grateful for Consejo Nacional de Ciencia y Tecnología and BEIFI fellowships. We thank to Luis Rendón (TEM and HRTEM), Marcela Guerrero Cruz (XRD) and Mario García (SEM/EDS) for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plascencia-Hernández, F., Valverde-Aguilar, G., Singh, N. et al. Photocatalytic hydrogen production from aqueous methanol solution using Pt nanocatalysts supported on mesoporous TiO2 hollow shells. J Sol-Gel Sci Technol 77, 39–47 (2016). https://doi.org/10.1007/s10971-015-3826-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3826-x