Abstract
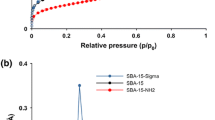
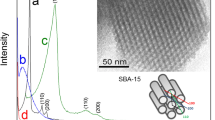
A mesoporous silica-based hybrid material composed of silica xerogel modified with an ionic silsesquioxane, which contains the 1,4-diazoniabicyclo[2.2.2]octane chloride group, was obtained. The silsesquioxane film is highly dispersed on the surface. This hybrid material was utilized to develop a carbon paste electrode (CPE) for determination of methyl parathion. Transmission FTIR, elemental analysis and N2 adsorption–desorption isotherms were used for characterization of the material. The electrochemical behavior of methyl parathion was evaluated by cyclic voltammetry and differential pulse voltammetry. It was observed a linear response to methyl parathion in the concentration range from 1.25 × 10−7 to 2.56 × 10−6 mol L−1 by employing the carbon paste electrode, in Britton–Robinson buffer solution (pH 6). The achieved detection limit (3 SD of the blank divided by the slope of calibration curve) was 0.013 µmol L−1 and sensitivity was 6.3 µA µmol L−1. This result shows the potentiality of this electrode for application as electrochemical sensor for methyl parathion.









Similar content being viewed by others
References
Rodrigues SR, Caldeira C, Castro BB, Gonçalves F, Nunes B, Antunes SC (2011) Pestic Biochem Physiol 99:181–188
Frenich AG, Vidal JLM, Sicilia ADC, Rodríguez MJG, Bolaños PP (2006) Anal Chim Acta 558:42–52
Upadhyay S, Rao GR, Sharma MK, Bhattacharya BK, Rao VK, Vijayaraghavan R (2009) Biosens Bioelectron 25:832–838
Videira RA, Antunes-Madeira AM, Loopes VICF, Madeira VMC (2011) Biochim Biophys Acta 1511:360–368
Reddy KG, Madhavi G, Swamy BEK, Reddy S, Reddy AVB, Madhavi V (2013) J Mol Liq 180:26–30
Vicente A, Yolanda P (2004) Trends Anal Chem 23:772–789
Hernandez F, Sancho JV, Pozo OJ (2005) Bioanal Chem 382:934–946
Nousiainen M, Perakorpi K, Sillanpaa M (2007) Talanta 72:984–990
Kumaravel A, Chandrasekaran M (2010) J Electroanal Chem 638:231–235
Tcheumi HL, Tonle IK, Ngameni E, Walcarius A (2010) Talanta 81:972–979
Liu G, Lin Y (2005) Electrochem Commun 7:339–343
Yazhen W, Hongwin Q, Siqian H, Junhui X (2010) Sens Actuators B 147:587–592
Jeyapragasam T, Saraswathi R, Chen S-M, Lou B-S (2013) Int J Electrochem Sci 8:12353–12366
Zeng Y, Yu D, Yu Y, Zhou T, Shi G (2012) J Hazard Mater 217–218:315–322
Ma J-C, Zhang W-D (2011) Microchim Acta 175:309–314
Zhao L, Zhao F, Zeng B (2013) Sens Actuators B 176:818–824
Sanghavi BJ, Hirsch G, Karna SP, Srivastava AK (2012) Anal Chim Acta 735:37–45
Raghu P, Swamy BEK, Reddy TM, Chandrashekar BN, Reddaiah K (2012) Bioelectrochemistry 83:19–24
Vuk AS, Jovanovski V, Pollet-Villard A, Jerman I, Orel B (2008) Solar Energy Mater Solar Cells 92:126–135
Gushikem Y, Benvenutti EV, Kholin Y (2008) Pure Appl Chem 80:1593–1611
Benvenutti EV, Moro CC, Costa TMH, Gallas MR (2009) Quim Nova 32:1926–1933
Lucho AMS, Pissetti FL, Gushikem Y (2004) J Colloid Interface Sci 275:251–256
Arenas LT, Gay DSF, Moro CC, Dias SLP, Azambuja DS, Costa TMH, Benvenutti EV, Gushikem Y (2008) Micropor Mesopor Mater 112:273–283
Arguello J, Magosso HA, Landers R, Gushikem Y (2008) J Electroanal Chem 617:45–52
Magosso HA, Luz RCS, Gushikem Y (2010) Electroanalysis 22:216–222
de Menezes EW, Nunes MR, Arenas LT, Dias SLP, Garcia ITS, Gushikem Y, Costa TMH, Benvenutti EV (2012) J Solid State Electrochem 16:3703–3713
Tamborim SM, Azambuja DS, Arenas LT, Costa TMH, Benvenutti EV (2008) Patente PI 0800519-2 (in Portuguese)
Nunes MR, Gushikem Y, Landers R, Dupont J, Costa TMH, Benvenutti EV (2012) J Sol-Gel Sci Technol 63:258–265
Arenas LT, Dias SLP, Moro CC, Costa TMH, Benvenutti EV, Lucho AMS, Gushikem Y (2006) J Colloid Interface Sci 297:244–250
Foschiera JL, Pizzolato TM, Benvenutti EV (2001) J Braz Chem Soc 12:159–164
Gregg SJ, Sing KSW (1982) Adsorption, surface area and porosity, chapters 3 and 4. Academic, London
Arenas LT, Langaro A, Gushikem Y, Moro CC, Benvenutti EV, Costa TMH (2003) J Sol–Gel Sci Technol 28:51–56
Ying JY, Benziger JB, Navrotsky A (1993) J Am Ceram Soc 76:2571–2582
Pavan FA, Franken L, Moreira CA, Costa TMH, Benvenutti EV, Gushikem Y (2001) J Colloid Interface Sci 241:413–416
Yang S, Luo S, Liu C, Wei W (2012) Colloids Surf B Biointerfaces 96:75–79
Arenas LT, Villis PCM, Arguello J, Landers R, Benvenutti EV, Gushikem Y (2010) Talanta 83:241–248
Dong J, Wang X, Qiao F, Liu P, Ai S (2013) Sens Actuators B 186:774–780
Li C, Wang Z, Zhan G (2011) Colloids Surf B 82:40–45
Xue R, Kang TF, Lu LP, Cheng SY (2012) Appl Surf Sci 258:6040–6045
Vidotti C, Carvalhal RF, Mendes RK, Ferreira DCM, Kubota LT (2011) J Braz Chem 22:3–20
Dahlin AB, Dielacher B, Rajendran P, Sugihara K, Sannomiya T, Zenobi-Wong M, Vörös J (2012) Anal Bioanal Chem 402:1773–1784
Acknowledgments
The authors are grateful to CNPQ (Conselho Nacional de Desenvolvimento Científico e Tecnológico), FAPERGS (Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul) and CAPES (Coordenação de Aperfeiçoamento Pessoal de Nível Superior) for their financial support and fellowships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caldas, E.M., de Menezes, E.W., Pizzolato, T.M. et al. Ionic silsesquioxane film immobilized on silica applied in the development of carbon paste electrode for determination of methyl parathion. J Sol-Gel Sci Technol 72, 282–289 (2014). https://doi.org/10.1007/s10971-014-3367-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-014-3367-8