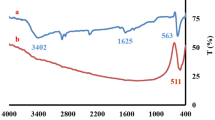
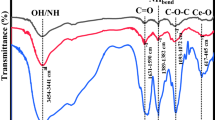
Abstract
In this paper, CeO2 and cobalt-doped CeO2 nanorods synthesized by surfactant free co-precipitation method. The microstructures of the synthesized products were characterized by XRD, FESEM and TEM. The structural properties of the grown nanorods have been investigated using electron diffraction and X-ray diffraction. High resolution transmission electron microscopy studies show the polycrystalline nature of the Co-doped cerium oxide nanorods with a length of about 300 nm and a diameter of about 10 nm were produced. The X-ray Photoelectron spectrum confirms the presence of cobalt in cerium oxide nanorods. From BET, the specific surface area of the CeO2 (Co-doped) nanostructures (131 m2 g−1) is found to be significantly higher than that of pure CeO2 (52 m2 g−1). The Co-doped cerium nanorods exhibit an excellent photocatalytic performance in rapidly degrading azodyes acid orange 7 (AO7) in aqueous solution under UV illumination.









Similar content being viewed by others
References
Gates B, Mayers B, Cattle B, Xia YN (2002) Adv Funct Mater 12:219–227
Li T, Yang SG, Huang LS, Gu BX, Du YW (2004) Nanotechnology 15:1479–1482
Huang Y, Duan XF, Wei QQ, Lieber CM (2001) Science 291:630–633
Arul NS, Mangalaraj D, Chen PC, Ponpandian P, Viswanathan C (2011) Mater Lett 65:2635–2638
Pan ZW, Dai ZR, Wang ZL (2001) Science 291:1947–1949
Zhang J, Jiang F, Zhang L (2004) J Phys Chem B 108:7002–7005
Murugan B, Ramaswamy AV (2007) J Am Chem Soc 129:3062–3063
Jasinski P, Suzuki T, Anderson HU (2003) Sens Actuators, B 95:73–77
Zhang YW, Si R, Liao CS, Yang CH, Xiao CX, Kou Y (2003) J. Phys. Chem. B 107:10159–10167
Pan C, Zhang D, Shi LJ (2008) Solid State Chem 181:1298–1306
Gao F, Lu Q, Komarneni SJ (2006) Nanosci Nanotechnol 6:3812–3819
Gu F, Wang Z, Han D, Shi C, Guo G (2007) Mater Sci Eng, B 139:62–68
Zhang D, Fu H, Shi L, Pan C, Li Q, Yu YC (2007) Inorg Chem 46:2446–2451
Wu GS, Xie G, Yuan XY, Cheng BC, Zhang LD (2004) Mater Res Bull 39:1023–1028
Tsunekawa S, Fukuda T, Kasuya A (2000) J Appl Phys 87:1318–1321
Mai MX, Sun LD, Zhang YW, Si R, Feng W, Zhang HP, Liu HC, Yan CH (2005) J Phys Chem B 109:24380–24385
Bugayeva N, Robinson J (2007) J Mater Sci Technol 23:237–241
Cushing BL, Kolesnichenko VL, Connor CLO (2004) J Chem Rev 104:3893–3946
Zawadzki MJ (2008) J Alloys Compd 454:347–351
Yan L, Xing X, Yu R, Qiao L, Chen J, Deng J, Liu G (2007) Scripta Mater 56:301–304
Yang J, Ferreira JMF (1998) Mater Res Bull 33:389–394
Tiejun C, Yuchao L, Zhenshan P, Yunfei L, Zongyuan W, Qian D (2009) J Environ Sci 21:997–1004
Zhang TM, Li J, Li H, Li Y, Shen W (2009) Catal Today 148:179–183
Zhang F, Chan S-W, Spanier JE, Apak E, Jin Q, Robinson RD, Irving Herman P (2002) Appl Phys Lett 80:127–129
May GJ (1978) J Mater Sci 13:261–267
Li L, Sasaki T, Shimizu Y, Koshizaki N (2009) J Phys Chem C 113:15948–15954
He Y, Li D, Xiao G, Chen W, Chen Y, Sun M, Huang H, Fu X (2009) J Phys Chem C 113:5254–5262
Ferrari V, Llois AM, Vildosola V (2010) J Phys: Condens Matter 22:276002–276010
Han WQ, Wu LJ, Zhu YM (2005) J Am Chem Soc 127:12814–12815
Elisangela F, Andrea Z, Fabio DG, Cristiano RM, Regina DL, Artur CP (2009) Int Biodeterior Biodegrad 63:280–288
Acknowledgments
One of the authors NSA would like to thank Lunghwa University for offering Internship program, Mr. Shun Cho and Ms. Koug Chen for their help in doing FESEM and Mr. Chih-Hua Yu for helping to perform TEM measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arul, N.S., Mangalaraj, D., Chen, P.C. et al. Enhanced photocatalytic activity of cobalt-doped CeO2 nanorods. J Sol-Gel Sci Technol 64, 515–523 (2012). https://doi.org/10.1007/s10971-012-2883-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-012-2883-7