Abstract
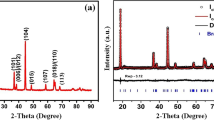
A lithium cobalt phosphate (LiCoPO4) cathode was synthesised by citric acid assisted sol–gel method and its electrochemical behaviour in alkaline secondary battery (using novel lithium hydroxide as the electrolyte) is reported. The sol–gel method using metal acetate precursors with citric acid as a chelating agent influenced the particle size and the homogeneity while yielding a single phase LiCoPO4 at a considerably lower temperature and shortened heating time, compared to that of the conventional solid state reaction. The cyclic voltammogram of LiCoPO4 showed a reversible redox process implying that de-intercalation and intercalation of lithium can occur in aqueous electrolyte. This was supported by X-ray diffraction (XRD) and Infra-red (IR) studies. The charge–discharge performance of the Zn/LiCoPO4 battery showed good capacity retention (after 25 cycles it delivered 90 % of its initial capacity). This enhanced capacity retention was attributed to the synergistic effect of particle homogeneity, reduced Li+ diffusion path and stability of the non-reactive aqueous electrolyte between the electrode and the electrolyte interface.







Similar content being viewed by others
References
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) J Electrochem Soc 144:1188–1194
Morgan D, Van der Ven A, Ceder G (2004) Electrochem Solid State Lett 7:A30–A32
Bramnik NN, Bramnik KG, Baehtz C, Ehrenberg H (2005) J Power Sour 145:74–81
Bramnik NN, Nikolowski K, Baehtz C, Bramnik KG, Ehrenberg H (2007) Chem Mater 19:908–915
Bramnik NN, Bramnik KG, Buhrmester T, Baehtz C, Ehrenberg H, Fuess H (2003) J Solid State Electrochem 8:558–564
Doan TNL, Taniguchi I (2011) J Power Sour 196:5679–5684
Satya Kishore MVVM, Varadaraju UV (2005) Mater Res Bull 40:1705–1712
Chen Z-Y, Zhu H-L, Ji S, Fakir R, Linkov V (2008) Solid State Ionics 179:1810–1815
Wang F, Yang J, NuLi Y, Wang J (2009) J Power Sour 196:4806–4810
Chung SY, Bloking JT, Chiang Y-M (2002) Nat Mater 1:123–128
Zhong S-K, Chen W, Li Y-H, Zou Z-G, Liu C-J (2010) Trans Nonferrous Met Soc China 20:s275–s278
Li W, Dahn JR, Wainwright DS (1994) Science 264:1115–1118
Beck F, Ruetschi P (2000) Electrochim Acta 45:2467–2482
Minakshi M (2010) Electrochim Acta 55:9174–9178
Minakshi M, Singh P, Sharma N, Blackford M, Ionescu M (2011) Ind Eng Chem Res 50:1899
Sathiyaraj K, Babu G, Bhuvaneswari D, Kalaiselvi N (2011) Ionics 17:49–59
Wang J, Liu X-M, Yang H, Shen X-D (2011) J Alloys Compd 509:712–718
Hsu K-F, Tsay S-Y, Hwang B-J (2004) J Mater Chem 14:2690–2695
Yang J, Xu J (2006) J Electrochem Soc 153:A716–A723
Minakshi M (2010) Electrochem Solid State Lett 3:A125–A127
Gangulibabu D, Bhuvaneswari N, Kalaiselvi N, Jayaprakash N, Periasamy P (2009) J Sol-Gel Sci Technol 49:137–144
Poovizhi PN, Selladurai S (2011) Ionics 17:13–19
Minakshi M, Sharma N, Ralph D, Appadoo D, Nallathamby K (2011) Electrochem Solid State Lett 14:A86–A89
Koleva V, Zhecheva E, Stoyanova R (2010) Eur J Inorg Chem 26:4091–4099
Burba CM, Frech R (2004) J Electrochem Soc 151:A1032–A1038
Ehrenberg H, Bramnik NN, Senyshyn A, Fuess H (2009) Solid State Sci 11:18–23
Osorio-Guillén JM, Holm B, Ahuja R, Johansson B (2004) Solid State Ionics 167:221–227
Acknowledgments
The author (M. M) wishes to acknowledge the Australian Research Council (ARC). This research was supported under ARC’s Discovery Projects funding scheme (DP1092543) and from the Australian Synchrotron Company Limited through the grant no AS103/HRIR3003. The views expressed herein are those of the authors and are not necessarily those of the Australian Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Minakshi, M., Kandhasamy, S. Influence of sol–gel derived lithium cobalt phosphate in alkaline rechargeable battery. J Sol-Gel Sci Technol 64, 47–53 (2012). https://doi.org/10.1007/s10971-012-2826-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-012-2826-3