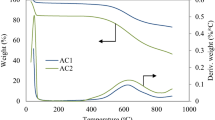
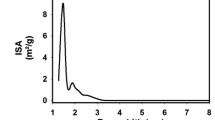
Abstract
Carbon xerogel (CX) was used for phenol adsorption from aqueous solution. CX was synthesized by sol–gel polycondensation of resorcinol with formaldehyde using sodium carbonate (Na2CO3) as catalyst. Then, it was dried by convective drying technique and pyrolyzed under inert atmosphere. Phenol adsorption kinetics was very fast, what was attributed to the presence of open pore structure. The kinetic studies showed that the adsorption process could be fitted to a pseudo-second-order model and the particle diffusion process is the rate-limiting step of the adsorption. The phenol removal was maximum and unaffected by pH changes when the initial pH of the phenol solution was in the range of 3–8. The optimum adsorbent dose obtained for phenol adsorption onto CX was 0.075 g/50 cm3 solution. The Langmuir model described the adsorption process better than the Freundlich isotherm model and the monolayer adsorption capacity is 32 mg g−1. Among the desorbing solutions used in this study, the most efficient desorbent was EtOH (100 %) which released about 87 % of phenol bound with the CX.













Similar content being viewed by others
References
Alkaram UF, Mukhlis AA, Al-Dujaili AH (2009) The removal of phenol from aqueous solutions by adsorption using surfactant-modified bentonite and kaolinite. J Hazard Mater 169:324–332
Singh KP, Malik A, Sinha S, Ojha P (2008) Liquid-phase adsorption of phenols using activated carbons derived from agricultural waste material. J Hazard Mater 150:626–641
Tor A, Cengeloglu Y, Ersoz M (2009) Increasing the phenol adsorption capacity of neutralized red mud by application of acid activation procedure. Desalination 242:19–28
Tor A, Cengeloglu Y, Aydin ME, Ersoz M (2006) Removal of phenol from aqueous phase by using neutralized red mud. J Colloid and Interface Sci 300:498–503
Hamdaoui O, Naffrechoux E (2007) Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon Part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J Hazard Mater 147:381–394
Liu QS, Zheng T, Wang P, Jiang JP, Li N (2010) Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem Eng J 157:348–356
Kumar A, Kumar S, Kumar S, Gupta DV (2007) Adsorption of phenol and 4-nitrophenol on granular activated carbon in basal salt medium: equilibrium and kinetics. J Hazard Mater 147:155–166
Senturk HB, Ozdes D, Gundogdu A, Duran C, Soylak M (2009) Removal of phenol from aqueous solutions by adsorption onto organomodified Tirebolu bentonite: equilibrium, kinetic and thermodynamic study. J Hazard Mater 172:353–362
Lin K, Pan J, Chen Y, Cheng R, Xu X (2009) Study the adsorption of phenol from aqueous solution on hydroxyapatite nanopowders. J Hazard Mater 161:231–240
Kuleyin A (2007) Removal of phenol and 4-chlorophenol by surfactant-modified natural zeolite. J Hazard Mater 144:307–315
Polat H, Molva M, Polat M (2006) Capacity and mechanism of phenol adsorption on lignite. Int J Miner Process 79:264–273
Lua AC, Jia Q (2009) Adsorption of phenol by oil–palm-shell activated carbons in a fixed bed. Chem Eng J 150:455–461
Dursun G, ÇiÇek H, Dursun AY (2005) Adsorption of phenol from aqueous solution by using carbonised beet pulp. J Hazard Mater B125:175–182
El-Naas MH, Al-Zuhair S, Alhaija MA (2010) Removal of phenol from petroleum refinery wastewater through adsorption on date-pit activated carbon. Chem Eng J 162:997–1005
Stavropoulos GG, Samaras P, Sakellaropoulos GP (2008) Effect of activated carbons modification on porosity, surface structure and phenol adsorption. J Hazard Mater 151:414–421
Meena AK, Mishra GK, Rai PK, Rajagopal C, Nagar PN (2005) Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J Hazard Mater 122:161–170
Wu X, Wu D, Fu R (2007) Studies on the adsorption of reactive brilliant red X-3B dye on organic and carbon aerogels. J Hazard Mater 147:1028–1036
Kadirvelu K, Goel J, Rajagopal C (2008) Sorption of lead, mercury and cadmium ions in multi-component system using carbon aerogel as adsorbent. J Hazard Mater 153:502–507
Lazarević S, Janković-Castvan I, Jovanović D, Milonjić S, Janaćković D, Petrović R (2007) Adsorption of Pb2+, Cd2+ and Sr2+ ions onto natural and acid-activated sepiolites. Appl Clay Sci 37:47–57
Boehm HP (1966) Advances in catalysis, vol 16. Academic Press, New York
Sharma CS, Kulkarni MM, Sharma A, Madou M (2009) Synthesis of carbon xerogel particles and fractal-like structures. Chem Eng Sci 64:1536–1543
Dong-hui L, Jie Z, Jun-he Y, Zi-jun H, Tong-qi L, Guo C, Ruil Z, Li-cheng L (2008) Preparation and microstructure control of carbon aerogels produced using m-cresol mediated sol-gel polymerization of phenol and furfural. New Carbon Mater 23:165–170
Stein A, Wang Z, Fierke MA (2009) Functionalization of porous carbon materials with designed pore architecture. Adv Mater 21:265–293
Guilminot E, Fischer F, Chatenet M, Rigacci A, Berthon-Fabry S, Achard P, Chainet E (2007) Use of cellulose-based carbon aerogels as catalyst support for PEM fuel cell electrodes: electrochemical characterization. J Power Sources 166:104–111
Lorjai P, Chaisuwan T, Wongkasemjit S (2009) Porous structure of polybenzoxazine-based organic aerogel prepared by sol–gel process and their carbon aerogels. J Sol-Gel Sci Technol 52:56–64
Du J, Song C, Zhao J, Zhu Z (2008) Effect of chemical treatment to hollow carbon nanoparticles (HCNP) on catalytic behaviors of the platinum catalysts. Appl Surf Sci 255:2989–2993
Sharma CS, Patil S, Saurabh S, Sharma A, Venkataraghavan R (2009) Resorcinol–formaldehyde based carbon nanospheres by electrospraying. Bull Mater Sci 32:39–246
Job N, Pirard R, Marien J, Pirard J-P (2004) Porous carbon xerogels with texture tailored by pH control during sol–gel process. Carbon 42:619–628
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquérol J, Siemieniewska T (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 57:603
Lee Y-F, Chang K-H, Chu C-Y, Chena H-L, Hu C–C (2011) Microstructure tuning of mesoporous silica prepared by evaporation-induced self-assembly processes: interactions among solvent evaporation, micelle formation/packing and sol condensation. RSC Adv 1:401–407
Sevilla M, Fuertes AB, Mokaya R (2011) High density hydrogen storage in superactivated carbons from hydrothermally carbonized renewable organic materials. Energy Environ Sci 4:1400–1410
Hameed BH, Rahman AA (2008) Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J Hazard Mater 160:576–581
Cheung WH, Szeto YS, McKay G (2007) Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour Technol 98:2897–2904
Dursun AY, Kalayci ÇS (2005) Equilibrium, kinetic and thermodynamic studies on the adsorption of phenol onto chitin. J Hazard Mater B123:151–157
Rodrigues LA, Silva MLCP, Alvarez-Mendes MO, Coutinho AR, Thim GP (2011) Phenol removal from aqueous solution by activated carbon produced from avocado kernel seeds. Chem Eng J 174:49–57
Alam MZ, Muyibi SA, Mansor MF, Wahid R (2007) Activated carbons derived from oil palm empty-fruit bunches: application to environmental problems. J Environ Sci 19:103–108
Yousef RI, El-Eswed B, Al-Muhtaseb AH (2011) Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: kinetics, mechanism, and thermodynamics studies. Chem Eng J 171:1143–1149
Acknowledgments
The authors gratefully acknowledge CAPES for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigues, L.A., Campos, T.M.B., Alvarez-Mendes, M.O. et al. Phenol removal from aqueous solution by carbon xerogel. J Sol-Gel Sci Technol 63, 202–210 (2012). https://doi.org/10.1007/s10971-012-2745-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-012-2745-3