Abstract
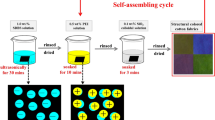
A new method for improving the anti-ultraviolet and anti-ageing abilities of wool fabric was reported in this paper. TiO2 sols and poly (sodium 4-styrene-sulfonate) (PSS) were coated on the wool fibers via layer-by-layer (LBL) electrostatic self-assembly deposition. The morphologies and compositions of TiO2 sol-coated wool fabrics were characterized using SEM, surface Zeta potential, apparent color depth (K/S), ultraviolet (UV) transmission and alkali solubility. The SEM pictures showed that there were quite a few deposits absorbed on the wool surface. The dyeing depth and Zeta potential presented obvious “layer–layer alternate vibration” along with the change of deposited materials, revealing the surface structure of the assembled wool fiber. The results of ultraviolet (UV) transmission and alkali solubility indicated that the modified wool fabrics obtained good anti-ultraviolet and anti-ageing properties. In addition, the sol-assembled wool fabrics had good washing fastness. The studies proved that the LBL electrostatic self-assembly deposition is a promising way to endow the textiles with surface functionality.








Similar content being viewed by others
References
Wang Q, Hauser PJ (2010) Developing a novel UV protection process for cotton based on layer-by-layer self-assembly. Carbohydr Polym 81(2):491–496
Wang Q, Gao XX, Fan XR et al (2010) Immobilization of lysozyme on wool surface via layer-by-layer electrostatic self-assembly and its antibacterial property. Acta Chim Sinica 68(20):2090–2103
Hyde GK, Park KJ, Stewart SM et al (2007) Atomic layer deposition of conformal inorganic nanoscale coatings on three-dimensional natural fiber systems: effect of surface topology on film growth characteristics. Langmuir 23(19):9844–9849
Decher G (1997) Fuzzy nanoasemblies: toward layered polymeirc multi-composites. Sci 277(5330):1232–1237
Ller RK (1966) Multilayers of colloidal particles. J. Colloid Interf Sci 21(6):569–594
Decher G, Hong JD, Schmitt J (1992) Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films 210(2):831–835
Sukhorulov GB, Donath E, Lichtenfeld H et al (1998) Layer-by-layer self assembly of polyelectrolytes on colloidal particles. Colloid Surf A 137(1):253–266
Donath E, Sukhorulov GB, Caruso F et al (1998) Novel hollow polymer shells by colloid templated assembly of polyelectrolytes. Angew Chem Int Edit 37(16):2201–2205
Sukhorulov GB, Donath E, Davis S et al (1998) Stepwise polyelectrolyte assembly on particle surfaces:a novel approach to colloid design. Polym Advan Technol 9(10–11):759–767
Lvov Y, Ariga K, Kunitake T et al (1995) Assembly of multicomponent protein films by means of electrostatic layer-by-layer adsorption. J Am Chem Soc 117(22):6117–6123
Decher G, Lvov Y, Schmitt J et al (1994) New nanocomposite films for biosensors: layer-by-layer adsorbed films of polyelectrolytes, proteins or DNA. Biosens Bioelectron 9(9–10):677–684
Serizawa T, Takeshita H, Akashi M (1998) Electrostatic adsorption of polystyrene nanospheres onto the surface of an ultrathin polymer film prepared by using an alternate adsorption technique. Langmuir 14(5):4088–4094
Caruso F, Caruso MA, Mohwald H (1998) Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Sci 282(5390):1111–1114
Caruso F, Möhwald H (1999) Preparation and characterization of ordered nanoparticle and polymer composite multilayers on colloids. Langmuir 15(23):8276–8281
Caruso F, Caruso RA, Mohwald H (1999) Produciton of hollow microspheres from nanostructured composite particles. Chem Mater 11(11):3309–3314
Caruso RA, Susha A, Caruso F (2001) Mulitlayered titania, silica and laponite nanoparticle coatings on polystyrene colloidal templates and resulting inorganic hollow spheres. Chem Mater 13(2):400–409
Caruso F, Shi XY, Caruso RA et al (2001) Hollow titania spheres from layered precursor deposition on sacirificila colloidal core particles. Adv Mater 13(10):740–744
Hao YZ, Yang MZ, Chen Y et al (1998) Charge transport properties of TiO2 nanocrystalline/nanoporous film. Acta Physico Chimica Sinica 14(4):309–313
Shen Z, Wang GT (1997) Colloid and surface chemistry. Chemical Industry Press, Beijing
Zhang HY, Zhang RP, Cai ZS (2008) Remedial action of transglutaminase on wool modification. Wool Text J Chin 36(2):11–14
Acknowledgments
This research was sponsored by National Natural Science Foundation of China (51073073), Qing Lan Project and Excellent Science and Technology Innovation Team in Colleges and Universities in Jiangsu Province Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1135), and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Wang, Q. & Fan, X.R. Layer-by-layer self-assembly of TiO2 sol on wool to improve its anti-ultraviolet and anti-ageing properties. J Sol-Gel Sci Technol 62, 338–343 (2012). https://doi.org/10.1007/s10971-012-2730-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-012-2730-x