Abstract
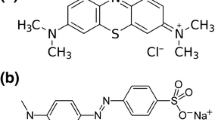
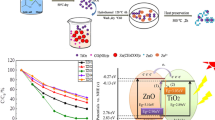
The Ni/TiO2 nanoparticles with different Ni dopant content were prepared by a modified sol–gel method. The structure and photoinduced charge properties of the as-prepared catalysts were determined using X-ray diffraction, transmission electron microscopy, UV–vis diffuse reflectance spectroscopy and surface photovoltage spectroscopy techniques, and the photocatalytic efficiency of these catalysts was tested using an organic dye. It was shown that Ni modification could greatly enhance the photocatalytic efficiency of these nanocomposite catalysts by taking the photodegradation of methyl orange as a model reaction. With appropriate ratio of Ni and TiO2, Ni/TiO2 nanocomposites showed the superior photocatalytic activity than the single TiO2 nanoparticles. Surface photovoltage spectra demonstrated that Ni modification could effectively inhibit the recombination of the photoinduced electron and holes of TiO2. This electron–hole pair separation conditions are responsible for the higher photocatalytic performance of Ni/TiO2 nanocomposites in the visible region of electromagnetic spectrum.






Similar content being viewed by others
References
Hoffmann MR, Martin ST, Choi W, Bahnemann DW (1995) Chem Rev 95:69
Schaaff TG, Blom DA (2002) Nano Lett 2:507
Sun B, Vorontsov AV, Smirniotis PG (2003) Langmuir 19:3151
Kim S, Choi W (2002) J Phys Chem B 106:13311
Einaga H, Ibusuki T, Futamura S (2004) Environ Sci Technol 38:285
Wang W, Zhang J, Chen F, Anpo M, He D (2010) Res Chem Intermed 36:163
Hao HY, He CX, Tian BZ, Zhang JL (2009) Res Chem Intermed 35:705
Yuan X, Zhang J, Anpo M, He D (2010) Res Chem Intermed 36:83
Xiong RC, Jia CG, Wei G (2002) J BUCT (Nature Science Edition) 29:34
Umebayashi T, Yamaki T, Itoh H, Asai K (2002) J Phys Chem Solids 63:1909
Sharma SD, Singh D, Saini KK, Kant C, Sharma V, Jain SC, Sharma CP (2006) Appl Catal A Gen 314:40
Sanchez E, Lopez T (1995) Mater Lett 25:271
Kronik L, Shapira Y (1999) Surf Sci Rep 37:1
Morikawa T, Irokawa Y, Ohwaki T (2006) Appl Catal A Gen 314:123
Litter MI (1999) Appl Catal B 23:89
Nakamura I, Negishi N, Kutsuna S, Ihara T, Sugihara S, Takeuchi K (2000) J Mol Catal A Chem 161:205
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities and Huazhong Agricultural University Scientific & Technological Self-innovation Foundation (2009QC016).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, D. Chemical synthesis of Ni/TiO2 nanophotocatalyst for UV/visible light assisted degradation of organic dye in aqueous solution. J Sol-Gel Sci Technol 58, 312–318 (2011). https://doi.org/10.1007/s10971-010-2393-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-010-2393-4