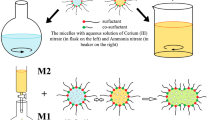
Abstract
In this research, CuO–ZrO2 nanoparticles are synthesized using microreactors made of surfactant/water/cyclohexane microemulsions. The effect of different microemulsion variables on the particle size and its distribution, such as water-to-surfactant molar ratio (W 0) and different surfactants are discussed. Three different surfactant types including cationic (CTAB), anionic (AOT), and nonionic (Brij56) are used. Also a different amount of water to surfactant in nano composite synthesis is used. The powders were characterized by DTA/TG, XRD, SEM, EDS, TEM and BET techniques and their physical properties are compared. The results show a decrease of particles size in presence of cationic surfactant. Narrow particles size distribution of the resultant CuO–ZrO2 nanocomposite in presence of cationic surfactant, anionic and nonionic surfactant is compared. Also for AOT surfactant, by raising water to surfactant molar ratio the particles size is increased and the optimum ratio is H2O: Surfactant = 0.32:0.055, respectively.









Similar content being viewed by others
References
Karimi S, Foulkes FR (2002) University of Toronto. Bull Sci Technol Soc 22(4):283–296
Cuciu C, Hoffmann AC, Vik A (2007) The effect of calcination and precursor proportion of YSZ nanoparticles obtained by modified sol–gel route. Department of Physics and Technology, University of Bergen Norway. Allegaten 55, Chemical Engineering Journal
Silva PD, Marchal N, Harle V, Cseri T, Kasztelan S (1998) Symposium on Recent Advances in Heteroatom Removal Presented Before the Divisionof Petroleum Chemistry Inc., 215th National Meeting, American Chemical Society, Dallas, TX
Daage M, Chianelli RR (1994) Structure-function relations in molybdenum sulfide catalysts: the "Rim-Edge" model. J Catal 149:414–427
Chianelli RR, Daage M (1989) Structure/function relations in transition metal sulfide catalysts. Stud Surf Sci Catal 50:1–19
Boutonnet M, Logdberg S, Elm Svensson E (2007) Recent developments in the application of nanoparticles prepared from W/O microemulsions in heterogeneous catalysis. Curr Opin Colloid Interface Sci 13(4):270–286
Chen Q, Shen X, Gao H (2007) Formation of nanoparticles in water-in-oil microemulsions controlled by the yield of hydrated electron: the controlled reduction of Cu2+. J Colloid Interface Sci 308:491–499
Brian L Cushing, Vladimir L Kolesnichenko, Charles JO’ Connor (2004) Recent advancesin the liquid-phase syntheses of inorganic nanoparticles, advanced materials research institute. University of New Orleans, New Orleans, Louisiana 70148-2820, Chem Rev 104, 3893–3946
Rosen MJ (1989) Surfactants and interfacial phenomena. Wiley, New York
Zhou R, Yu T, Jiang X, Chen F, Zheng X (1999) Temperature-programmed reduction and temperature-programmed desorption studies of CuO/ZrO2 catalyst. Appl Surf Sci 148:263–270
Dongare MK, Ramaswamy V, Gopinath CS, Ramaswamy AV, Scheurell S, Brueckner M, Kemnitz E (2001) Oxidation activity and 18O-Isotope Exchange Behavior of Cu-Stabilized Cubic Zirconia. J Catal 199:209–216
Vahidshad Y, Abdizadeh H, Bahrvandi HR, Baseri MA (2009) Structural and morphology of nanopowders copper-stabilized zirconia. Surf Rev Lett 16(4):569–577
Ghiaci M, Aghaei H, Abbaspur A (2007) Size-controlled synthesis of ZrO2–TiO2 nanoparticles prepared via reverse micelle method: investigation of particle size effect on the catalytic performance in vapor phase Beckmann rearrangement. Mat Res Bull 43(5):1255–1262
Nassar NN, Husein MM (2007) Effect of microemulsion variables on copper oxide nanoparticle uptake by AOT microemulsions. J Colloid Interface Sci 316:442–450
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vahidshad, Y., Abdizadeh, H., Akbari Baseri, M. et al. Size-controlled synthesis of CuO–ZrO2 nanoparticles prepared through reverse micelle method. J Sol-Gel Sci Technol 53, 263–271 (2010). https://doi.org/10.1007/s10971-009-2086-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-009-2086-z