Abstract
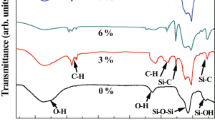
Hydrophobic silica aerogels have been prepared using the rapid supercritical extraction (RSCE) technique. The RSCE technique is a one-step methanol supercritical extraction method for producing aerogel monoliths in 3 to 8 h. Standard aerogels were prepared from a tetramethoxysilane (TMOS) recipe with a molar ratio of TMOS:MeOH:H2O:NH4OH of 1.0:12.0:4.0:7.4 × 10−3. Hydrophobic aerogels were prepared using the same recipe except the TMOS was replaced with a mixture of TMOS and one of the following organosilane co-precursors: methytrimethoxysilane (MTMS), ethyltrimethoxysilane (ETMS), or propyltrimeth-oxysilane (PTMS). Results show that, by increasing the amount of catalyst and increasing gelation time, monolithic aerogels can be prepared out of volume mixtures including up to 75% MTMS, 50% ETMS or 50% PTMS in 7.5–15 h. As the amount of co-precursor is increased the aerogels become more hydrophobic (sessile tests with water droplets yield contact angles up to 155°) and less transparent (transmission through a 12.2-mm thick sample decreases from 83 to 50% at 800 nm). The skeletal and bulk density decrease and the surface area increases (550–760 m2/g) when TMOS is substituted with increasing amounts of MTMS. The amount of co-precursor does not affect the thermal conductivity. SEM imaging shows significant differences in the nanostructure for the most hydrophobic surfaces.









Similar content being viewed by others
References
Pierre AC, Pajonk GM (2002) Chemistry of aerogels and their applications. Chem Rev 102:4243–4265
Akimov YK (2003) Fields of application of aerogels (review). Instrum Exper Tech 46(3):287–299
Miner M, Hosticka B, Norris P (2004) The effects of ambient humidity on the mechanical properties and surface chemistry of hygroscopic silica aerogel. J Non-Cryst Solids 350:285–289
Rao AP, Rao AV, Bangi UKH (2008) Low thermal conductive, transparent and hydrophobic ambient pressure dried silica aerogels with various preparation conditions using sodium silicate solutions. J Sol–Gel Sci Technol 47(1):85–94
Hüsing N, Schwertfeger F, Tappert W, Schubert U (1995) Influence of supercritical drying fluid on structure and properties of organically modified silica aerogels. J Non-Cryst Solids 186:37–43
Martín L, Ossó JO, Ricart S, Roig A, García O, Sastre R (2008) Organo-modified silica aerogels and implications for material hydrophobicity and mechanical properties. J Mater Chem 18(2):207–213
Yokogawa H, Yokoyama M (1995) Hydrophobic silica aerogel. J Non-Cryst Solids 186:23–29
Lee KH, Kim SY, Yoo KP (1995) Low-density, hydrophobic aerogels. J Non-Cryst Solids 186:18–22
Smirnova I, Suttiruengwong S, Arlt W (2004) Feasibility study of hydrophilic and hydrophobic silica aerogels as drug delivery systems. J Non-Cryst Solids 350:54–60
Lee C, Kim G, Hyun S (2002) Synthesis of silica aerogels from waterglass via new modified ambient drying. J Mater Sci 37(11):2237–2241
Rao AP, Rao AV, Pajonk GM (2005) Hydrophobic and physical properties of the two step processed ambient pressure dried silica aerogels with various exchanging solvents. J Sol–Gel Sci Technol 36(3):285–292
Schwertfeger F, Glaubitt W, Schubert U (1992) Hydrophobic aerogels from Si(OMe)4/MeSi(OMe)3 mixtures. J Non-Cryst Solids 145:85–89
Schwertfeger F, Hüsing N, Schubert U (1994) Influence of the nature of organic groups on the properties of organically modified silica aerogels. J Sol–Gel Sci Technol 2(1):103–108
Rao AV, Haranath D (1999) Effect of methyltrimethoxysilane as a synthesis component on the hydrophobicity and some physical properties of silica aerogels. Microporous Mesoporous Mater 30(2–3):267–273
Rao AV, Pajonk GM (2001) Effect of methyltrimethoxysilane as a co-precursor on the optical properties of silica aerogels. J Non-Cryst Solids 285(1–3):202–209
Štandeker S, Novak Z, Knez Ž (2007) Adsorption of toxic organic compounds from water with hydrophobic silica aerogels. J Colloid Interface Sci 310(2):362–368
Reynolds JG, Coronado PR, Hrubesh LW (2001) Hydrophobic aerogels for oil-spill clean up––synthesis and characterization. J Non-Cryst Solids 292(1–3):127–137
Hrubesh L, Coronado P, Satcher J (2001) Solvent removal from water with hydrophobic aerogels. J Non-Cryst Solids 285(1–3):328–332
Roig A, Molins E, Rodríguez E, Martínez S, Moreno-Mañas M, Vallribera A (2004) Superhydrophobic silica aerogels by fluorination at the gel stage. Chem Comm 2004(20):2316–2317
Gauthier BM, Bakrania SD, Anderson AM, Carroll MK (2004) A fast supercritical extraction technique for aerogel fabrication. J Non-Cryst Solids 350:238–243
Gauthier BM, Anderson AM, Bakrania SD, Mahony MK, Bucinell RB (2008) Method and device for fabricating aerogels and aerogel monoliths obtained thereby. U.S. Patent 7,384,988. 10 June 2008
Anderson AM, Wattley CW, Carroll MK (2009) Silica aerogels prepared via rapid supercritical extraction: effect of process variables on aerogel properties. J Non-Cryst Solids 355(2):101–108
Roth TB, Anderson AM, Carroll MK (2008) Analysis of a rapid supercritical extraction aerogel fabrication process: prediction of thermodynamic conditions during processing. J Non-Cryst Solids 354(31):3685–3693
Rao AV, Kulkarni M, Pajonk G, Amalnerkar D, Seth T (2003) Synthesis and characterization of hydrophobic silica aerogels using trimethylethoxysilane as a co-precursor. J Sol–Gel Sci Technol 27(2):103–109
Fidalgo A, Ciriminna R, Ilharco LM, Pagliaro M (2005) Role of the alkyl-alkoxide precursor on the structure and catalytic properties of hybrid sol–gel catalysts. Chem Mater 17:6686–6694
Acknowledgements
The Union College Aerogel Fabrication, Characterization, and Applications Laboratory has been funded by grants from the National Science Foundation (NSF MRI CTS-0216153, NSF RUI CHE-0514527, NSF MRI CMMI-0722842, and NSF RUI CHE-0847901), the American Chemical Society’s Petroleum Research Fund (ACS PRF 39796-B10), the Union College Faculty Research Fund and Union College Student Internal Education Fund. ECG received a Davenport Summer Research Fellowship from Union College. The SEM instrument was funded through grants from the National Science Foundation (NSF MRI 0619578) and New York State Assembly RESTORE-NY. The authors are grateful to Professor Brad Bruno for the use of the camera employed for contact angle measurements and helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anderson, A.M., Carroll, M.K., Green, E.C. et al. Hydrophobic silica aerogels prepared via rapid supercritical extraction. J Sol-Gel Sci Technol 53, 199–207 (2010). https://doi.org/10.1007/s10971-009-2078-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-009-2078-z