Abstract
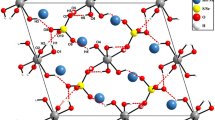
The molecular alkoxide, ErNb2(OPri)13 was prepared by metathesis with 2KNb(OPri)6, KOPri and ErCl3, and structurally determined by single-crystal X-ray diffraction techniques. Each molecule contains a central Er3+-ion coordinated by six OPri groups of which one is terminal. On each side is an Nb5+-ion coordinated by six OPri groups placed, one via a double alkoxo-bridge and the other via a triple alkoxo-bridge. It is isostructural with LaNb2(OPri)13, in spite of the large difference in ionic radius between Er3+ and La3+ (16%). The compound was characterized by its IR- and UV-vis-NIR-spectroscopic fine structures, and found to be structurally intact in hexane:isopropanol solution.
Similar content being viewed by others
References
C.D. Chandler, C., Rogers, and M.J. Hampden-Smith, Chem. Rev. 93, 1205 (1993).
C.J. Brinker and G.W. Scherer, The Physics and Chemistry of Sol-Gel Processing (Academic Press, Inc., London, 1990).
M. Wijk, R. Norrestam, M. Nygren, G. Westin, Inorg. Chem. 35, 1077 (1996).
G. Westin, Å. Ekstrand, L. Börjesson, and E. Zanghellini, J. Phys. Chem. Solids 61, 61 (2000); M. Wijk, E. Wikstad M. Kritikos R. Norrestam G. Westin, long abstract and poster in 5th World congress of the Institute of American Chemical Engineers, San Diego 1996; Sol-Gel 95, Faro, Poster presentation.
C.K. Ryu, H. Choi K. Kim, Appl. Phys. Lett. 66, 2496 (1995).
E.P. Turevskaya, N.Ya. Turova, A.V. Korolev A.I. Yanovsky, and Yu.T. Struchkov, Polyhedron 14, 1531 (1995).
STOE, X-SHAPE Revision 1.09. Crystal Optimisation for Numerical Absorption Correction: (Darmstadt, 1997).
G.M. Sheldrick, Acta Cryst. Sect. A 46, 467 (1994).
G.M. Sheldrick (Ed.), SHELX97. Computer Program for the Refinement of Crystal Structures, Release 97-2 ed. (University of Göttingen, Germany, Göttingen, 1997).
R.C. Mehrotra, M.M. Aggrawal P.N. Kapoor, J. Chem. Soc. 2673 (1968).
S. Mishra, U.M. Tripathi A. Singh R.C. Mehrotra, J. Chin. Chem. Soc. 49, 335 (2002).
G. Westin, M. Kritikos M. Wijk, J. Solid State Chem. 141, 168 (1998).
M. Kritikos, M. Moustiakimov M. Wijk G. Westin, RCS Dalton Trans. 13, 1931 (2001).
G. Westin, M. Moustiakimov M. Kritikos, Inorg. Chem. 41, 3249 (2002).
G. Westin and M. Kritikos, J. Sol-Gel Sci. Technol. 26, 115 (2003).
K.B. Yatsimirskii and N.K. Davidenko, Coord. Chem. Rev. 27, 223 (1979).
C. Görller-Walrand and K. Binnemans, Handbook on the Physics and Chemistry of Rare Earths, edited by K.A. Gschneidner Jr. and L. Eyring (Elsevier Science, B.V 1998), Chapter 167, p. 101.
G. Westin, R. Norrestam, M. Nygren, and M. Wijk, J. Solid State Chem. 135, 149 (1998).
M. Kritikos, M. Wijk, and G. Westin, Acta. Cryst. Sect. C. 54, 576 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kritikos, M., Westin, G. Synthesis, Structure and Properties of ErNb2(OPri)13. J Sol-Gel Sci Technol 32, 25–29 (2004). https://doi.org/10.1007/s10971-004-5759-7
Issue Date:
DOI: https://doi.org/10.1007/s10971-004-5759-7