Abstract
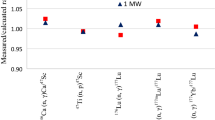
The present work was planned to evaluate the reactor production of 47Sc from calcium and titanium targets at Egyptian Second Research Reactor (ETRR-2) based on 46Ca (n, γ) 47Ca \(\mathop{\longrightarrow}\limits^{\beta - }\) 47Sc and 47Ti (n, p) 47Sc nuclear ractions, respectively. A comparison between the two routs in terms of specific activity of 47Sc and co-produced radioimpurities was carried out. The MCNPX2.7.0 code was used to estimate the levels of all radionuclides produced throughout as well as after the irradiation of 46CaCO3, natTiO2 and 47TiO2 targets. The computational results were verified by experimental measurements, and a good aggrement was found between both. Additionally, our results showed that 47Sc production is greatly preferred from titanium route rather than calcium route.




Similar content being viewed by others
References
Stöcklin G, Qaim SM, Rösch F (1995) The impact of radioactivity on medicine. Radiochim Acta 70(71):249–272
Qaim SM, Scholten B, Neumaier B (2018) New developments in the production of theranostic pairs of radionuclides. J Radioanal Nucl Chem 318(3):1493–1509
Srivastava SC (2011) Paving the way to personalized medicine: production of some theragnostic radionuclides at Brookhaven National Laboratory. Radiochim Acta 99(10):635–640
Yordanova A, Eppard E, Kürpig S, Bundschuh RA, Schoenberger S, Gonzalez-Carmona M, Essler M (2017) Theranostics in nuclear medicine practice. Oncotargets Therapy 10:4821
Chakravarty R, Chakraborty S, Ram R, Dash A (2017) An electroamalgamation approach to separate 47Sc from neutron-activated 46Ca target for use in cancer theranostics. Sep Sci Technol 52(14):2363–2371
Herzog H, Rosch F, Stocklin G, Lueders C, Qaim SM, Feinendegen LE (1993) Measurement of pharmacokinetics of Yttrium-86 radiopharmaceuticals with PET and radiation. J Nucl Med 34:2222–2226
Rösch F, Herzog H, Qaim SM (2017) The beginning and development of the theranostic approach in nuclear medicine, as exemplified by the radionuclide pair 86Y and 90Y. Pharmaceuticals 10(2):56
Qaim SM (2019) Theranostic radionuclides: recent advances in production methodologies. J Radioanal Nucl Chem 322(3):1257–1266
Gizawy MA, Mohamed NM, Aydia MI, Soliman MA, Shamsel-Din HA (2020) Feasibility study on production of Sc-47 from neutron irradiated Ca target for cancer theranostics applications. Radiochim Acta 108(3):207–215
Pietrelli L, Mausner LF, Kolsky KL (1992) Separation of carrier-free 47Sc from titanium targets. J Radioanal Nucl Chem 157(2):335–345
Majkowska-pilip A, Bilewicz A (2011) Macrocyclic complexes of scandium radionuclides as precursors for diagnostic and therapeutic radiopharmaceuticals. J Inorg Biochem 105(2):331
Müller C, Bunka M, Haller S, Köster U, Groehn V, Bernhardt P, Schibli R (2014) Promising prospects for 44Sc-/47Sc-based theragnostics: application of 47Sc for radionuclide tumor therapy in mice. J Nucl Med 55(10):1658–1664
Domnanich KA, Müller C, Benešová M, Dressler R, Haller S, Köster U, Ponsard B, Schibli R, Türler A, van der Meulen NP (2017) 47Sc as useful β–-emitter for the radiotheragnostic paradigm: a comparative study of feasible production routes. EJNMMI Radiopharm Chem 2(1):5
Gizawy MA, Aydia MI, Monem IMA, Shamsel-Din HA, Siyam T (2019) Radiochemical separation of reactor produced Sc-47 from natural calcium target using poly(acrylamide-acrylic acid)/multi-walled carbon nanotubes composite. Appl Radiat Isot 150:87–94
Yagi M, Kondo K (1977) Preparation of carrier-free 47Sc by the 48Ti(γ, p) reaction. Int J Appl Radiat Isot 28:463–468
Kopecky P, Szelecsényi F, Molnair T, Mikecz P, Tárkányi F (1993) Excitation functions of (p, xn) reactions on natTi: monitoring of bombarding proton beams. Appl Radiat Isot 44:687
Kolsky KL, Joshi V, Mausner LF, Srivastava SC (1998) Radiochemical purification of no-carrier-added scandium-47 for radioimmunotherapy. Appl Radiat Isot 49(12):1541–1549
Mausner LF, Kolsky KL, Joshi V, Srivastava SC (1998) Radionuclide development at BNL for nuclear medicine therapy. Appl Radiat Isot 49:285–294
Bokhari TH, Mushtaq A, Khan IU (2010) Separation of no-carrier-added radioactive scandium from neutron irradiated titanium. J Radioanal Nucl Chem 283(2):389–393
Bartoś B, Majkowska A, Kasperek A, Krajewski S, Bilewicz A (2012) New separation method of no-carrier added 47Sc from titanium targets. Radiochim Acta 100:457–461
Mamtimin M, Harmon F, Starovoitova VN (2015) Sc-47 production from titanium targets using electron linacs. Appl Radiat Isot 102:1–4
Starovoitova VN, Cole PL, Grimm TL (2015) Accelerator-based photoproduction of promising beta-emitters 67Cu and 47Sc. J Radioanal Nucl Chem 305(1):127–132
Rane S, Harris JT, Starovoitova VN (2015) 47Ca production for 47Ca/47Sc generator system using electron linacs. Appl Radiat Isot 97:188–192
Deilami-nezhad L, Moghaddam-Banaem L, Sadeghi M, Asgari M (2016) Production and purification of Scandium-47: a potential radioisotope for cancer theranostics. Appl Radiat Isot 118:124–130
Rotsch DA, Brown MA, Nolen JA, Brossard T, Henning WF, Chemerisov SD, Greene J (2018) Electron linear accelerator production and purification of scandium-47 from titanium dioxide targets. Appl Radiat Isot 131:77–82
Gizawy MA, Shamsel-Din HA, Abdelmonem IM, Ibrahim MI, Mohamed LA, Metwally E (2020) Synthesis of chitosan-acrylic acid/multiwalled carbon nanotubes composite for theranostic 47Sc separation from neutron irradiated titanium target. Int J Biol Macromol 163:79–86
Pelowitz DB (2011) MCNPX user’s manual version 2.7. 0-LA-CP-11-00438. Los Alamos National Laboratory
Hosseini SF, Sadeghi M, Aboudzadeh MR (2017) Theoretical assessment and targeted modeling of TiO2 in reactor towards the scandium radioisotopes estimation. Appl Radiat Isot 127:116–121
Mandour A, Megahid RM, Hassan MH, Abd El Salam TM (2007) Characterization and application of the thermal neutron radiography beam in the Egyptian Second Experimental and Training Research Reactor (ETRR-2). Science and Technology of Nuclear Installations
Hassanain AM, Mohamed NM, Aly MN, Badawi AA, Gaheen MA (2011) Neutron flux characterization for radioisotope production at ETRR-2. World Acad Sci Eng Technol Int J Math Comput Phys Electr Comput Eng 5(1):36–40
Mohamed NM, Gaheen MA (2016) Design of fast neutron channels for topaz irradiation. Nucl Eng Des 310:429–437
Lamarch JR (1983) Introduction to nuclear engineering. Addison-Wesley, Berlin
Mohamed NMA (2017) Accurate corrections of HPGe detector efficiency for NAA samples. J Radiat Nucl Appl 2:37–43
Soliman MA, Mohamed NM, Takamiya K, Sekimoto S, Inagaki M, Oki Y, Ohtsuki T (2020) Estimation of 47 Sc and 177 Lu production rates from their natural targets in Kyoto University Research Reactor. J Radioanal Nucl Chem 2020:1–9
Loveless CS, Blanco JR, Diehl GL, Elbahrawi RT, Carzaniga TS, Braccini S, Lapi SE (2020) Cyclotron production and separation of scandium radionuclides from natural titanium metal and titanium dioxide targets. J Nucl Med 62:131–136
Acknowledgements
This work was supported by the IAEA Research Contract No: 20548 in the form of IAEA CRP on ‘‘Therapeutic Radiopharmaceuticals Labelled with New Emerging Radionuclides “(67Cu, 186Re, 47Sc)”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gizawy, M.A., Mohamed, N. Theoretical and experimental investigations of Sc-47 production at Egyptian Second Research Reactor (ETRR-2). J Radioanal Nucl Chem 328, 1–7 (2021). https://doi.org/10.1007/s10967-021-07620-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07620-3