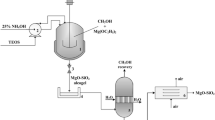
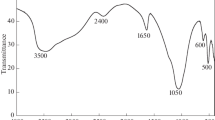
Abstract
Adsorption of 75Se(IV) oxyanions onto hydrous zirconium oxide (HZO) is investigated. At pHs 2.2 and 5.3, most of Se(IV) are removed within 5 min and the equilibrium is attained at 40 min. Kinetic and equilibrium data are analyzed by several kinetic and isotherm models, respectively. Adsorption of Se(IV) at different pHs (1.5–12) is dependent on its initial concentration. HZO exhibited a high adsorption capacity for Se(IV), Qmax = 213.19 mg/g, which is higher than those reported for other adsorbents. Thermodynamic studies revealed that the present adsorption process is feasible, spontaneous and endothermic. Adsorption of Se(IV) in presence of foreign anions is also evaluated.











Similar content being viewed by others
References
Lawson S, Macy JM (1995) Bioremediation of selenite in oil refinery wastewater. Appl Microbiol Biotechnol 43:762–765
Lemly AD (2004) Aquatic selenium pollution is a global environmental safety issue. Ecotoxicol Environ Saf 59:44–56
Mavrov V, Stamenov S, Todorova E, Chmiel H, Erwe T (2006) New hybrid electrocoagulation membrane process for removing selenium from industrial wastewater. Desalination 201:290–296
Torres J, Pintos V, Domínguez S, Kremer C, Kremer E (2010) Selenite and selenate speciation in naturalwaters: interaction with +2 metal ions. J Solut Chem 39:1–10
Ashworth DJ, Shaw G (2006) Soil migration, plant uptake and volatilisation of radio-selenium from a contaminated water table. Sci Total Environ 370:506–514
Jang M, Min SH, Kim TH, Park JK (2006) Removal of arsenite and arsenate using hydrous ferric oxide incorporated into naturally occurring porous diatomite. Environ Sci Technol 40:1636–1643
Ren Z, Zhang G, Chen JP (2011) Adsorptive removal of arsenic from water by an iron–zirconium binary oxide adsorbent. J Colloid Interface Sci 358:230–237
Bortun A, Bortun M, Pardini J, Khainakov SA, Garcı JR (2010) Synthesis and characterization of a mesoporous hydrous zirconium oxide used for arsenic removal from drinking water. Mater Res Bull 45:142–148
Dixit S, Hering JG (2013) Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: implications for arsenic mobility. Environ Sci Technol 37:4182–4189
Kim SS, Min JH, Lee JK, Baik MH, Choi JW, Shin HS (2012) Effects of pH and anions on the sorption of selenium ions onto magnetite. J Environ Radioact 104:1–6
Rodrigues LA, Maschio LJ, da Silva R, da Silva MLCP (2010) Adsorption of Cr(VI) from aqueous solution by hydrous zirconium oxide. J Hazard Mater 173:630–636
Dou X, Mohan D Jr., Pittman CU, Yang S (2012) Remediating fluoride from water using hydrous zirconium oxide. Chem Eng J 198–199:236–245
Suzuki TM, Tanco ML, Tanaka DAP, Matsunaga H, Yokoyama T (2001) Adsorption characteristics and removal of oxo-anions of arsenic and selenium on the porous polymers loaded with monoclinic hydrous zirconium oxide. Sep Sci Technol 36:103–111
Weerasekara NA, Choo KH, Choi SJ (2013) Metal oxide enhanced microfiltration for the selective removal of Co and Sr ions from nuclear laundry wastewater. J Membr Sci 447:87–95
Lin J, Zhan Y, Wang H, Chu M, Wang C, He Y, Wang X (2017) Effect of calcium ion on phosphate adsorption onto hydrous zirconium oxide. Chem Eng J 309:118–129
Bortun A, Bortun M, Pardini J, Khainakov SA, Garcı JR (2010) Effect of competitive ions on the arsenic removal by mesoporous hydrous zirconium oxide from drinking water. Mater Res Bull 45:1628–1634
Su Y, Cui H, Li Q, Gao S, Shang JK (2013) Strong adsorption of phosphate by amorphous zirconium oxide nanoparticles. Water Res 47:5018–5026
Lazarevic S, Jankovic-Castvan I, Jovanovic D, Milonjic S, Janackovic D, Petrovic R (2007) Adsorption of Pb2+, Cd2+ and Sr2+ ions onto natural and acid-activated sepiolites. Appl Clay Sci 37:47–57
Mahmoud MR, Soliman MA, Allan KF (2014) Adsorption behavior of samarium(III) from aqueous solutions onto PAN@SDS core-shell polymeric adsorbent. Acta, Radiochim. https://doi.org/10.1515/ract-2014-2299
Mahmoud MR, Seliman AF (2014) Evaluation of silica/ferrocyanide composite as a dual-function material for simultaneous removal of 137Cs+ and 99TcO4 − from aqueous solutions. Appl Radiat Isot 91:141–154
Mahmoud MR, Soliman MA, Allan KF (2014) Removal of Thoron and Arsenazo III from radioactive liquid waste by sorption onto cetyltrimethylammonium-functionalized polyacrylonitrile. J Radioanal Nucl Chem 300:1195–1207
Moreno-Pirajan JC, Giraldo L (2011) Activated carbon obtained by pyrolysis of potato peel for the removal of heavy metal copper(II) from aqueous solutions. J Anal Appl Pyrolysis 90:42–47
Tuzen M, Sari A (2010) Biosorption of selenium from aqueous solution by green algae (Cladophora hutchinsiae) biomass: equilibrium, thermodynamic and kinetic studies. J Chem Eng 158:200–206
Caliskan N, Kul AR, Alkan S, Sogut EG, Alacabey I (2011) Adsorption of Zinc(II) on diatomite and manganese-oxide-modified diatomite: a kinetic and equilibrium study. J Hazard Mater 193:27–36
Rodrigues LA, Maschio LJ, Coppio LSC, Thim GP, da Silva MLCP (2012) Adsorption of phosphate from aqueous solution by hydrous zirconium Oxide. Environ Technol 33:1345–1351
Cui W, Li P, Wang Z, Zheng S, Zhang Y (2018) Adsorption study of selenium ions from aqueous solutions using MgO nanosheets synthesized by ultrasonic method. J Hazard Mater 34:268–276
Ma Z, Shan C, Liang J, Tong M (2018) Efficient adsorption of selenium(IV) from water by hematite modified magnetic nanoparticles. Chemosphere 193:134–141
Lu Z, Yu J, Zeng H, Liu Q (2017) Polyamine-modified magnetic graphene oxide nanocomposite for enhanced selenium removal. Sep Purf Technol 183:249–257
Hamed MM, Holiel M, El-Aryan YF (2017) Removal of selenium and iodine radionuclides from waste solutions using synthetic inorganic ion exchanger. J Mol Liquids 242:722–731
Soliman MA, Mahmoud MR, Ali AH, Othman SH (2016) The sorption mechanism of selenium-75 on Amberlite MB9L. J Radioanal Nucl Chem 307:567–575
Xiao W, Yan B, Zeng H, Liu Q (2016) Dendrimer functionalized graphene oxide for selenium removal. Carbon 105:655–664
Kongsri S, Janpradit K, Buapa KA, Techawongstien S, Chanthai S (2013) Nanocrystalline hydroxyapatite from fish scale waste: preparation, characterization and application for selenium adsorption in aqueous solution. Chem Eng J 215–216:522–532
Zelmanov G, Semiat R (2013) Selenium removal from water and its recovery using iron (Fe3+) oxide/hydroxide-based nanoparticles sol (NanoFe) as an adsorbent. Sep Purf Technol 103:167–172
Han DS, Batchelor B, Abdel-Wahab A (2011) Sorption of selenium(IV) and selenium(VI) to mackinawite (FeS): effect of contact time, extent of removal, sorption envelopes. J Hazard Mater 186:451–457
Hasan SH, Ranjan D, Talat M (2010) Agro-industrial waste ‘wheat bran’ for the biosorptive remediation of selenium through continuous up-flow fixed-bed column. J Hazard Mater 181:1134–1142
Bleiman N, Mishael YG (2010) Selenium removal from drinking water by adsorption to chitosan–clay composites and oxides: batch and columns tests. J Hazard Mater 183:590–595
Zhang L, Liu N, Yang L, Lin Q (2009) Sorption behavior of nano-TiO2 for theremoval of selenium ions from aqueous solution. J Hazard, Mater, p 170
Hang C, Li Q, Gao S, Shang K (2012) As(III) and As(V) Adsorption by hydrous zirconium oxide nanoparticles synthesized by a hydrothermal process followed with heat treatment. Ind Eng Chem Res 51:353–361
Acknowledgements
The authors would like to thank all the staffs of Nuclear Chemistry Department and Egypt Second Research Reactor at Egyptian Atomic Energy Authority.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rashad, G.M., Soliman, M.A. & Mahmoud, M.R. Removal of radioselenium oxyanions from aqueous solutions by adsorption onto hydrous zirconium oxide. J Radioanal Nucl Chem 317, 593–603 (2018). https://doi.org/10.1007/s10967-018-5916-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-5916-z