Abstract
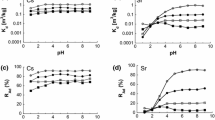
Adsorption and retardation of Sr2+ by four clays were investigated, which were evaluated by using the distribution coefficient (K D ) and average penetration length ratio of Sr2+ to water (r). K D values for Sr2+ among the four clay minerals were in the order: kaolinite < illite < vermiculite ≪ allophane, and Sr2+ penetration length ratio (r) followed the inverse order. Adsorption was further analyzed by using Langmuir adsorption model for competitive Sr2+ and Na+ adsorption at constant NaCl concentration. Conditional affinity constants suggested that Sr2+ adsorption on the variable charge sites of clay edges contributed to the high conditional Sr2+ affinities.





Similar content being viewed by others
References
Sureda R, Martínez-Lladó X, Rovira M et al (2010) Sorption of strontium on uranyl peroxide: implications for a high-level nuclear waste repository. J Hazard Mater 181:881–885. https://doi.org/10.1016/j.jhazmat.2010.05.095
İnan S, Altaş Y (2010) Adsorption of strontium from acidic waste solution by Mn–Zr mixed hydrous oxide prepared by Co-precipitation. Sep Sci Technol 45:269–276. https://doi.org/10.1080/01496390903409666
Bondarkov MD, Oskolkov BY, Gaschak SP et al (2011) Environmental radiation monitoring in the Chernobyl exclusion zone—history and results 25 years after. Health Phys 101:442–485. https://doi.org/10.1097/HP.0b013e318229df28
Kaçan E, Kütahyali C (2012) Adsorption of strontium from aqueous solution using activated carbon produced from textile sewage sludges. J Anal Appl Pyrolysis 97:149–157. https://doi.org/10.1016/j.jaap.2012.06.006
Steinhauser G, Schauer V, Shozugawa K (2013) Concentration of Strontium-90 at selected Hot Spots in Japan. PLoS ONE 8:e57760. https://doi.org/10.1371/journal.pone.0057760
Yusan S, Sema E (2011) Adsorption characterization of Strontium on PAN/Zeolite composite adsorbent. World J Nucl Sci Technol 1:6–12. https://doi.org/10.4236/wjnst.2011.11002
Erten HN, Aksoyoglu S, Hatipoglu S, Gokturk H (1988) Sorption of cesium and strontium on montmorillonite and kaolinite. Radiochim Acta 44–45:185. https://doi.org/10.1524/ract.1988.4445.1.147
Elvan B, Atun G (2006) Adsorption behavior of strontium on binary mineral mixtures of Montmorillonite and Kaolinite. Appl Radiat Isot 64:957–964. https://doi.org/10.1016/j.apradiso.2006.03.008
Mahoney JJ, Langmuir D (1991) Adsorption of Sr to kaolinite, illite and montmorillonite at high ionic strengths. Radiochim Acta 54:139–144. https://doi.org/10.1524/ract.1991.54.3.139
Bilgin B, Atun G, Keçeli G (2001) Adsorption of strontium on illite. J Radioanal Nucl Chem 250:323–328. https://doi.org/10.1023/A:1017960015760
Parkman RH, Charnock JM, Livens FR, Vaughan DJ (1998) A study of the interaction of strontium ions in aqueous solution with the surfaces of calcite and kaolinite. Geochim Cosmochim Acta 62:1481–1492. https://doi.org/10.1016/S0016-7037(98)00072-6
Rani RD, Sasidhar P (2012) Geochemical and thermodynamic aspects of sorption of strontium on kaolinite dominated clay samples at Kalpakkam. Environ Earth Sci 65:1265–1274. https://doi.org/10.1007/s12665-011-1374-4
Keçeli G (2015) Adsorption kinetics and equilibria of Strontium onto kaolinite adsorption kinetics and equilibria of Strontium onto kaolinite. Sep Sci Technol 50:72–78. https://doi.org/10.1080/01496395.2014.955206
Ning Z, Ishiguro M, Koopal LK et al (2017) Strontium adsorption and penetration in kaolinite at low Sr2+concentration. Soil Sci Plant Nutr 63:14–17. https://doi.org/10.1080/00380768.2016.1277435
Bors J, Grony A, Dultz S (1997) Iodide, caesium and strontium adsorption by organophilic vermiculite. Clay Miner 32:21–28. https://doi.org/10.1180/claymin.1997.032.1.04
Ivanets AI, Prozorovich VG, Kouznetsova TF et al (2016) Mesoporous manganese oxides prepared by sol-gel method: synthesis, characterization and sorption properties towards strontium ions. Environ Nanotechnol Monit Manag 6:261–269. https://doi.org/10.1016/j.enmm.2016.11.004
Ivanets AI, Prozorovich VG, Kouznetsova TF et al (2018) Sorption behavior of 85Sr onto manganese oxides with tunnel structure. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-018-5771-y
Oleksiienko O, Levchuk I, Sitarz M et al (2015) Removal of strontium (Sr2 +) from aqueous solutions with titanosilicates obtained by the sol-gel method. J Colloid Interface Sci 438:159–168. https://doi.org/10.1016/j.jcis.2014.09.075
Wahlberg JS, Baker JH, Vernon RW, Dewar RS (1965) Exchange adsorption of Strontium on clay minerals. Geol Surv Bull 1140-C
Su CM, Harsh JB (1993) The electrophoretic mobility of allophane and imogolite in the presence of inorganic anions and citrate. Clays Clay Miner 41:461–471. https://doi.org/10.1346/CCMN.1993.0410407
Schulze DG (2002) An introduction to soil mineralogy. In: Soil mineralogy with environmental applications
Bourikas K, Vakros J, Kordulis C, Lycourghiotis A (2003) Potentiometric mass titrations: experimental and theoretical establishment of a new technique for determining the Point of Zero Charge (PZC) of metal (Hydr) Oxides. J Phys Chem B 107:9441–9451. https://doi.org/10.1021/jp035123v
Heidmann I, Christl I, Leu C, Kretzschmar R (2005) Competitive sorption of protons and metal cations onto kaolinite: experiments and modeling. J Colloid Interface Sci 282:270–282. https://doi.org/10.1016/j.jcis.2004.08.019
Abollino O, Giacomino A, Malandrino M, Mentasti E (2008) Interaction of metal ions with montmorillonite and vermiculite. Appl Clay Sci 38:227–236. https://doi.org/10.1016/j.clay.2007.04.002
Fuller AJ, Shaw S, Peacock CL et al (2016) EXAFS study of Sr sorption to Illite, goethite, chlorite, and mixed sediment under hyperalkaline conditions. Langmuir 32:2937–2946. https://doi.org/10.1021/acs.langmuir.5b04633
Rafferty P, Shiao S-Y, Binz CM, Meyer RE (1981) Adsorption of Sr(II) on clay minerals: effects of salt concentration, loading, and pH. J Inorg Nucl Chem 43:797–805. https://doi.org/10.1016/0022-1902(81)80224-2
Bunde RL, Rosentreter JJ, Liszewski MJ et al (1997) Effects of calcium and magnesium on strontium distribution coefficients. Environ Geol 32:219–229. https://doi.org/10.1007/s002540050210
Jeong CH (2001) Mineralogical and hydrochemical effects on adsorption removal of Cesium-137 and Strontium-90 by kaolinite. J Environ Sci Heal Part A 36:1089–1099. https://doi.org/10.1081/ESE-100104133
Malandrino M, Abollino O, Giacomino A et al (2006) Adsorption of heavy metals on vermiculite: influence of pH and organic ligands. J Colloid Interface Sci 299:537–546. https://doi.org/10.1016/j.jcis.2006.03.011
Missana T, Garcia-Gutierrez M, Alonso U (2008) Sorption of strontium onto illite/smectite mixed clays. Phys Chem Earth 33:156–162. https://doi.org/10.1016/j.pce.2008.10.020
Bolt G (1978) Transport and accumulation of soluble soil components. In: Bolt GH, Bruggenwert MGM (eds) Soil chemistry: a basic elements. Elsevier, Amsterdam, pp 126–140
Ohashi F, Wada S-I, Suzuki M et al (2002) Synthetic allophane from high-concentration solutions: nanoengineering of the porous solid. Clay Miner 37:451–456. https://doi.org/10.1180/0009855023730052
Delgado AV, Gonzalez-Caballero F, Hunter RJ et al (2007) Measurement and interpretation of electrokinetic phenomena. J Colloid Interface Sci 309:194–224. https://doi.org/10.1016/j.jcis.2006.12.075
Kuroda Y, Nakaishi K (2003) Setting velocity and structure of kaolinite floc in sodium chloride solution. Clay Sci 12:103–107. https://doi.org/10.11362/jcssjclayscience1960.12.103
Wada S, Wada K (1977) Density and structure of allophane. Clay Miner 12:289–298
Totten MW, Hanan MA, Knight D, Borges J (2002) Characteristics of mixed-layer smectite/illite density separates during burial diagenesis. Am Mineral 87:1571–1579
Bruijn RHC, Burg J-P (2012) Chemical compaction of illite shale: an experimental study. PhD thesis, Utrecht University, the Netherland
Folorunso O (2015) Microwave processing of vermiculite. PhD thesis, University of Nottingham
Maeda T, Soma K (1986) Physical properties. In: Wada K (ed) Ando soils in Japan. Kyushu University Press, Fukuoka, pp 99–123
Kitagawa Y (1971) Unit particle of allophane. Am Mineral 56:465–475
Sposito G (1989) The chemistry of soils. Oxford University Press, New York
Evangelou V (1998) Environmental soil and water chemistry: principle and applications. Wiley, New York
Iyoda F, Hayashi S, Arakawa S et al (2012) Applied clay science synthesis and adsorption characteristics of hollow spherical allophane nano-particles. Appl Clay Sci 56:77–83. https://doi.org/10.1016/j.clay.2011.11.025
Nakao A, Thiry Y, Funakawai S, Kosaki T (2008) Characterization of the frayed edge site of micaceous minerals in soil clays influenced by different pedogenetic conditions in Japan and northern Thailand. Soii Sci Plant Nutr 54:479–489. https://doi.org/10.1111/j.1747-0765.2008.00262.x
Gualtieri AF, Ferrari S, Leoni M et al (2008) Structural characterization of the clay mineral illite-1M. J Appl Crystallogr 41:402–415. https://doi.org/10.1107/S0021889808004202
Tayor B, Ashcroft G (1972) Physical edaphology. W.H. Freeman and Company, San Francisco
Baver L, Gardner W, Gardner WR (1972) Soil physics, 4th edn. Wiley, New York
Wang L, Maes A, De Canniere P, Van Der Lee J (1998) Sorption of europium on illite (Silver Hill Montana). Radiochim Acta 82:233–238. https://doi.org/10.1524/ract.1998.82.special-issue.233
Adel G, Naoto M, Azza E, Teruo H (2007) Charge characteristics of nano-ball allophane as affected by zinc adsorption. J Appl Sci 7:103–108. https://doi.org/10.3923/jas.2007.103.108
Hayes KF, Redden G, Ela W, Leckie JO (1991) Surface complexation models: an evaluation of model parameter estimation using FITEQL and oxide mineral titration data. J Colloid Interface Sci 142:448–469. https://doi.org/10.1016/0021-9797(91)90075-J
Hiemstra T, van Riemsdijk WH (1996) A surface structural approach to ion adsorption: the charge distribution (CD) model. J Colloid Interface Sci 179:488–508. https://doi.org/10.1006/jcis.1996.0242
Lagaly G, Mecking O, Penner D (2001) Colloidal magnesium aluminum hydroxide and heterocoagulation with a clay mineral. II. Heterocoagulation with sodium montmorillonite. Colloid Polym Sci 279:1097–1103. https://doi.org/10.1007/s003960100526
Morimoto K, Anraku S, Hoshino J et al (2012) Surface complexation reactions of inorganic anions on hydrotalcite-like compounds. J Colloid Interface Sci 384:99–104. https://doi.org/10.1016/j.jcis.2012.06.072
Gu X, Evans LJ (2007) Modelling the adsorption of Cd(II), Cu(II), Ni(II), Pb(II), and Zn(II) onto Fithian illite. J Colloid Interface Sci 307:317–325. https://doi.org/10.1016/j.jcis.2006.11.022
Mekhamer WK, Assaad FF (1999) Thermodynamics of Sr–Mg vermiculite exchange and the effect of PVA on Mg release. Thermochim Acta 334:33–38. https://doi.org/10.1016/S0040-6031(99)00123-9
Van Olphen H (1963) An introduction to clay colloid chemistry. Wiley, New York
Ishiguro M (1992) Ion transport in soil with ion exchange reaction: effect of distribution ratio. Soil Sci Soc Am J 56:1738. https://doi.org/10.2136/sssaj1992.03615995005600060013x
Acknowledgements
The authors would like to express gratitude to the China Scholarship Council (CSC) for supporting Zigong Ning’s overseas study. This research was further supported by a Grant-in-Aid for Scientific Research (No. 25252042) from the Japan Society for the Promotion of Science. The authors also thank Dr. Shin-Ichiro Wada of Kyushu University for his valuable support in the preparation of synthesized allophane.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ning, Z., Ishiguro, M., Koopal, L.K. et al. Comparison of strontium retardation for kaolinite, illite, vermiculite and allophane. J Radioanal Nucl Chem 317, 409–419 (2018). https://doi.org/10.1007/s10967-018-5870-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-5870-9