Abstract
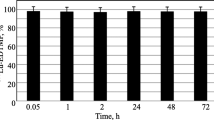
Zoledronic acid is a bisphosphonates to treat bone problems. Rhenium-188-zoledronic acid could be used as a palliate agent in skeletal metastases. Radiolabeled product was prepared through optimized radiochemical procedures. The organs uptake especially bone uptake assessed in healthy animals mice. The radiochemical purity of the radiotracer was > 95% which was stable up to 24 h. Bone uptake (1.08 ± 0.14% ID/g at 1 h) with retention of activity in bone up to 24 h (0.26 ± 0.06% ID/g) was reached. This radiotracer can be used as a developing candidate for a palliative treatment of bone metastasis.







Similar content being viewed by others
References
Vincent J, Vigorita MD (2007) Cancer-principles and practice of oncology. Lippincott, Williams & Wilkins, Philadelphia, pp 2713–2729
Mundy GR (2002) Metastasis: metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2(8):584–593
Mantyh PW (2014) The neurobiology of skeletal pain. Eur J Neurosci 39:508–519
Diel IJ (2006) What do patients with metastatic bone pain need? Eur J Cancer Suppl 4(8):1–3
Moos R, Costa L, Ripamonti CI, Niepel D, Santini D (2017) Improving quality of life in patients with advanced cancer: targeting metastatic bone pain. Eur J Cancer 71:80–94
Sartor O, Hoskin P, Bruland ØS (2013) Targeted radio-nuclide therapy of skeletal metastases. Cancer Treat Rev 39:18–26
Iwamoto J, Takeda T, Ichimura S (2002) Transient relief of metastatic cancer bone pain by oral administration of etidronate. J Bone Miner Metab 20(4):228–234
Green JR (2004) Bisphosphonates: preclinical review. Oncologist 9(Suppl 4):3–13
Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO (2003) Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol 21(23):4277–4284
Vitale G, Fonderico F, Martignetti A, Caraglia M, Ciccarelli A, Nuzzo V, Bbruzzese A, Lupoli G (2001) Pamidronate improves the quality of life and induces clinical remission of bone metastases in patients with thyroid cancer. Br J Cancer 84(12):1586–1590
Tripathy D, Lichinitzer M, Lazarev A, MacLachlan SA, Apffelstaedt J, Budde M, Bergstrom B (2004) Oral ibandronate for the treatment of metastatic bone disease in breast cancer: efficacy and safety results from a randomized, double-blind, placebo-controlled trial. Ann Oncol 15(5):743–750
Reid IR, Miller P, Lyles K, Fraser W, Brown JP, Saidi Y, Mesenbrink P, Su G, Pak J, Zelenakas K, Luchi M, Richardson P, Hosking D (2005) Comparison of a single infusion of zoledronic acid with risedronate for Paget’s disease. N Engl J Med 353(9):898–908
Kohno N, Aogi K, Minami H, Nakamura S, Asaga T, Iino Y, Watanabe T, Goessl C, Ohashi Y, Takashima S (2005) Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol 23(15):3314–3321
Wardley A, Davidson N, Barrett-Lee P, Hong A, Mansi J, Dodwell D, Murphy R, Mason T, Cameron DW (2005) Zoledronic acid significantly improves pain scores and quality of life in breast cancer patients with bone metastases: a randomised, crossover study of community vs hospital bisphosphonate administration. Br J Cancer 92(10):1869–1876
Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, Apffelstaedt J, Hussein M, Coleman RE, Reitsma DJ, Seaman JJ, Chen BL, Ambros Y (2001) Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J 7(5):377–387
Lange R, Ter Heine R, Knapp RF, de Klerk JM, Bloemendal HJ, Hendrikse NH (2016) Pharmaceutical and clinical development of phosphonate-based radiopharmaceuticals for the targeted treatment of bone metastases. Bone 91:159–179
Hoskin P, Sartor O, O’Sullivan JM, Johannessen DC, Helle SI, Logue J, Bottomley D, Nilsson S, Vogelzang NJ, Fang F, Wahba M, Aksnes AK, Parker C (2014) Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 5ALSYMPCA6 trial. Lancet Oncol 15(12):1397–1406
Morris MJ, Sartor O, Vogelzang NJ, Shore ND, Cislo P, Bangerter K, Sweeney C (2015) Effect of radium-223 dichloride (Ra-223) on pain from US EAP. J Clin Oncol 33:A160
Pandit-Taskar N, Batraki M, Divgi CR (2004) Radiopharmaceutical therapy for palliation of bone pain from osseous metastases. J Nucl Med 45:1358–1365
Pillai MRA, Dash A, Knapp FF (2012) Rhenium-188: availability from the 188W/188Re generator and status of current applications. Curr Radiopharm 5:228–243
Sartor O, Reid RH, Hoskin PJ, Quick DP, Ell PJ, Coleman RE, Kotler JA, Freeman LM, Olivier P (2004) Samarium-153-lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology 63:940–945
Das T, Shinto A, Karuppuswamy Kamaleshwaran K, Banerjee S (2016) Theranostic treatment of metastatic bone pain with 177Lu-DOTMP. Clin Nucl Med 41(12):966–967
Baum RP, Kulkarni HR (2012) THERANOSTICS: from molecular imaging using Ga-68 labeled tracers and PET/CT to personalized radionuclide therapy-the bad berka experience. Theranostics 2:437–447
Paes FM, Serafini AN (2010) Systemic metabolic radiopharmaceutical therapy in the treatment of metastatic bone pain in Seminars in nuclear medicine. Semin Nucl Med 40(2):89–104
Argyrou M, Valassi A, Andreou M, Lyra M (2013) Rhenium-188 production in hospitals, by W-188/Re-188 generator, for easy use in radionuclide therapy. Int J Mol Imaging 2013:290750
Liepe K, Kropp J, Runge R, Kotzerke J (2003) Therapeutic efficiency of rhenium-188-HEDP in human prostate cancer skeletal metastases. Br J Cancer 89(4):625–629
Meckel M, Bergmann R, Miederer M, Roesch F (2016) Bone targeting compounds for radiotherapy and imaging: *Me(III)-DOTA conjugates of bisphosphonic acid, pamidronic acid and zoledronic acid. EJNMMI Radiophar Chem 1(1):14
Kochetova T, Krylov V, Smolyarchuk M, Sokov D, Lunev A, Shiryaev S, Kruglova O, Makeenkova T, Petrosyan K, Dolgova A, Poluektova M, Galkin V, Kaprin A (2017) 188Re zoledronic acid in the palliative treatment of painful bone metastases. Int J Nucl Medi Res. https://doi.org/10.15379/2408-9788.2017.08
Erfani M, Rahmani N, Doroudi A, Shafiei M (2017) Preparation and evaluation of rhenium-188-pamidronate as a palliative treatment in bone metastasis. Nucl Med Biol 49:1–7
Janoki GA, Polyak A, Kiraly R, Balogh L, Korosi L, Mathe D (2007) Comparative evaluation of therapeutic radiopharmaceuticals. International Atomic Energy Agency, Vienna
Erfani M, Doroudi A, Dinari MA, Shirmardi SP (2015) Preparation of a rhenium-188 labeled bisphosphonate for bone pain palliation therapy. J Radioanal Nucl Chem 303:2027–2032
Stenbeck G, Horton MA (2000) A new specialized cell-matrix interaction in actively resorbing osteoclasts. J Cell Sci 13:1577–1587
Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Golub E, Rodan GA (1991) Bisphosphonate action: alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest 88(6):2095–2105
Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RG, Ebetino FH (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38(5):617–627
Acknowledgements
We like to appreciate Mr. Pezham for providing sodium perrehenate and help through animal study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erfani, M., Tabatabaei, M., Doroudi, A. et al. Radiolabeling of zoledronic acid with 188Re as a new palliative agent radiotracer in treatment of bone tumors. J Radioanal Nucl Chem 316, 491–500 (2018). https://doi.org/10.1007/s10967-018-5781-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-5781-9