Abstract
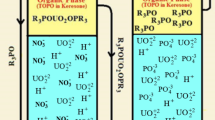
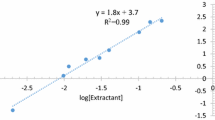
A kinetic study of the uranium transport from the sulfate medium through the bulk liquid membrane was conducted by Alamine 336 as a carrier. The effects of the sulfuric acid and uranium concentration in the donor phase, (NH4)2CO3 concentration in the acceptor phase and carrier concentration in the liquid membrane phase on the uranium transport were investigated. Maximum transport (83.12%) was obtained at 0.0125 mol L−1 Alamine 336 in the liquid membrane phase, 0.5 mol L−1 (NH4)2CO3 in the acceptor phase and 0.15 mol L−1 H2SO4 and 50 mg L−1 uranium in the donor phase after 24 h.






Similar content being viewed by others
References
Kim JS, Han KS, Kim SJ, Kim S-D, Lee J-Y, Han C, Kumar JR (2016) Synergistic extraction of uranium from Korean black shale ore leach liquors using amine with phosphorous based extractant systems. J Radio Nucl Chem 307(2):843–854
Ladeira ACQ, Morais CA (2005) Uranium recovery from industrial effluent by ion exchange—column experiments. Miner Eng 18(13–14):1337–1340
Mellah A, Chegrouche S, Barkat M (2006) The removal of uranium (VI) from aqueous solutions onto activated carbon: kinetic and thermodynamic investigations. J Colloid Interface Sci 296(2):434–441
Gu B, Liang L, Dickey M, Yin X, Dai S (1998) Reductive precipitation of uranium (VI) by zero-valent iron. Environ Sci Technol 32(21):3366–3373
Gorby YA, Lovley DR (1992) Enzymic uranium precipitation. Environ Sci Technol 26(1):205–207
Roach JD, Zapien JH (2009) Inorganic ligand-modified, colloid-enhanced ultrafiltration: a novel method for removing uranium from aqueous solution. Water Res 43(18):4751–4759
Shen J, Schäfer A (2014) Removal of fluoride and uranium by nanofiltration and reverse osmosis: a review. Chemosphere 117:679–691
Bhalara PD, Punetha D, Balasubramanian K (2014) A review of potential remediation techniques for uranium (VI) ion retrieval from contaminated aqueous environment. J Environ Chem Eng 2(3):1621–1634
Kumar JR, Kim J-S, Lee J-Y, Yoon H-S (2011) A brief review on solvent extraction of uranium from acidic solutions. Sep Purif Rev 40(2):77–125
Sole KC, Cole PM, Feather AM, Kotze MH (2011) Solvent extraction and ion exchange applications in Africa’s resurging uranium industry: a review. Solvent Extr Ion Exch 29(5–6):868–899
Soldehoff K (2006) Solvent extraction and ion exchange technologies for uranium recovery from saline solutions. In: ALTA uranium conference
Uranium extraction technology (1993) IAEA, (International Atomic Energy Agency). Austria, Vienna
Jooste M, Kotze M, Auerswald D (2010) The advantages of true continuous counter-current elution in uranium processing. In: Proceedings of ALTA uranium conference, Perth, Australia
Taylor A (2008) Review of CIX and RIP systems for uranium extraction. In: ALTA Uranium. ALTA Metallurgical Services, Melbourne
Benedict M, Pigford TH, Levi HW (1981) Nuclear chemical engineering. McGraw Hill, New York
Chia DE, Cooper WC (1986) An alternate process for the recovery of uranium by ion exchange: a study of the HIMIX process. Hydrometallurgy 16(1):1–25
Kislik VS (2009) Liquid membranes: principles and applications in chemical separations and wastewater treatment. Elsevier, Amsterdam
De Agreda D, García Díaz I, López Gómez FA, Alguacil FJ (2011) Supported liquid membranes technologies in metals removal from liquid effluents. Rev Met. https://doi.org/10.3989/revmetalmadrid.1062
Zaheri P, Abolghasemi H, Mohammadi T, Maraghe MG (2015) Dysprosium pertraction through facilitated supported liquid membrane using D2EHPA as carrier. Chem Pap 69(2):279–290
Allahyari SA, Ahmadi SJ, Minuchehr A, Charkhi A (2017) Th(IV) recovery from aqueous waste via hollow fiber renewal liquid membrane (HFRLM) in recycling mode: modelling and experimental validation. RSC Adv 7(12):7413–7423
Allahyari SA, Minuchehr A, Ahmadi SJ, Charkhi A (2016) Th (IV) transport from nitrate media through hollow fiber renewal liquid membrane. J Membr Sci 520:374–384
Allahyari SA, Minuchehr A, Ahmadi SJ, Charkhi A (2017) Thorium pertraction through hollow fiber renewal liquid membrane (HFRLM) using Cyanex 272 as carrier. Prog Nucl Energy 100:209–220
Chaturabul S, Srirachat W, Wannachod T, Ramakul P, Pancharoen U, Kheawhom S (2015) Separation of mercury (II) from petroleum produced water via hollow fiber supported liquid membrane and mass transfer modeling. Chem Eng J 265:34–46
Fouad EA (2017) Optimizing emulsion liquid membrane process for extraction of nickel from wastewater using Taguchi method. Int J Res Sci 3(1):1–7
Zaheri P, Davarkhah R (2017) Rapid removal of uranium from aqueous solution by emulsion liquid membrane containing thenoyltrifluoroacetone. J Environ Chem Eng 5(4):4064–4068
Breembroek G, Van Straalen A, Witkamp G, Van Rosmalen G (1998) Extraction of cadmium and copper using hollow fiber supported liquid membranes. J Membr Sci 146(2):185–195
Raghuraman BJ, Tirmizi NP, Kim B-S, Wiencek JM (1995) Emulsion liquid membranes for wastewater treatment: equilibrium models for lead and cadmium di-2-ethylhexyl phosphoric acid systems. Environ Sci Technol 29(4):979–984
Raut D, Mohapatra P (2013) Non-dispersive solvent extraction of uranium from nitric acid medium by several amides and their mixture with TODGA using a hollow fiber contactor. Sep Sci Technol 48(16):2436–2443
Zaheri P, Mohammadi T, Abolghasemi H, Maraghe MG (2015) Supported liquid membrane incorporated with carbon nanotubes for the extraction of Europium using Cyanex272 as carrier. Chem Eng Res Des 100:81–88
Zahakifar F, Charkhi A, Torab-Mostaedi M, Davarkhah R (2017) Performance evaluation of hollow fiber renewal liquid membrane for extraction of uranium (VI) from acidic sulfate solution. Radiochim Acta. https://doi.org/10.1515/ract-2017-2821
Kocherginsky N, Yang Q, Seelam L (2007) Recent advances in supported liquid membrane technology. Sep Purif Technol 53(2):171–177
El Sayed MS (2003) Uranium extraction from gattar sulfate leach liquor using aliquat-336 in a liquid emulsion membrane process. Hydrometallurgy 68(1–3):51–56
Hirato T, Kishigami I, Awakura Y, Majima H (1991) Concentration of uranyl sulfate solution by an emulsion-type liquid membrane process. Hydrometallurgy 26(1):19–33
Biswas S, Rupawate V, Hareendran K, Roy S (2014) Transport of U (VI) from sulphuric acid medium across supported liquid membrane (SLM) containing di-(2-ethylhexyl) phosphoric acid (D2EHPA)/n-dodecane as a carrier. J Radio Nucl Chem 299(3):1199–1207
Shamsipur M, Davarkhah R, Khanchi AR (2010) Facilitated transport of uranium (VI) across a bulk liquid membrane containing thenoyltrifluoroacetone in the presence of crown ethers as synergistic agents. Sep Purif Technol 71(1):63–69
Shamsipur M, Davarkhah R, Yamini Y, Hassani R, Reza Khanchi A (2009) Selective facilitated transport of uranium (VI) across a bulk liquid membrane containing benzoyltrifluoroacetone as extractant-carrier. Sep Sci Technol 44(11):2645–2660
Davarkhah R, Khanramaki F, Asgari M, Salimi B, Ashtari P, Shamsipur M (2013) Kinetic studies on the extraction of uranium (VI) from phosphoric acid medium by bulk liquid membrane containing di-2-ethylhexyl phosphoric acid. J Radio Nucl Chem 298(1):125–132
Ramkumar J, Nayak S, Maiti B (2002) Transport of uranyl ion across a bulk liquid membrane using calixarene and synergistic agents as carriers. J Membr Sci 196(2):203–210
Nanda D, Oak M, Maiti B, Chauhan H, Dutta P (2002) Selective and uphill transport of uranyl ion in the presence of some base metals and thorium across bulk liquid membrane by di (2-ethylhexyl) phosphoric acid. Sep Sci Technol 37(14):3357–3367
Zhang W, Liu J, Ren Z, Wang S, Du C, Ma J (2009) Kinetic study of chromium (VI) facilitated transport through a bulk liquid membrane using tri-n-butyl phosphate as carrier. Chem Eng J 150(1):83–89
Diaconu I, Ruse E, Aboul-Enein HY, Bunaciu AA (2016) Analytical applications of transport through bulk liquid membranes. Crit Rev Anal Chem 46(4):332–341
Chang SH (2016) Types of bulk liquid membrane and its membrane resistance in heavy metal removal and recovery from wastewater. Des Water Treat 57(42):19785–19793
Južnič K, Fedina Š (1974) The extraction of uranium (IV) from sulphuric acid by tri-octylamine in benzene. Microchim Acta 62(1):39–44
Lyle SJ, Tamizi M (1983) A study of equilibria in the extraction of uranium (VI) from aqueous sulphate solution by tri-n-octylamine in benzene or petroleum spirit. Hydrometallurgy 11(1):1–11
Kumar JR, Kim J-S, Lee J-Y, Yoon H-S (2010) Solvent extraction of uranium (VI) and separation of vanadium (V) from sulfate solutions using Alamine 336. J Radio Nucl Chem 285(2):301–308
Morais CA, Gomiero LA (2005) Uranium stripping from tertiary amine loaded solution by ammonium sulfate. Miner Eng 18(13):1277–1281
Amaral JC, Morais CA (2010) Thorium and uranium extraction from rare earth elements in monazite sulfuric acid liquor through solvent extraction. Miner Eng 23(6):498–503
Avelar É, Alvarenga C, Resende G, Morais C, Mansur M (2017) modeling of the solvent extraction equilibrium of uranium sulfate with Alamine 336. Braz J Chem Eng 34(1):355–362
Khanramaki F, Shirani A, Safdari J, Torkaman R (2017) Investigation of liquid extraction and thermodynamic studies on uranium from sulfate solution by Alamine 336 as an extractant. Int J Environ Sci Technol 125:181–189
Ramadevi G, Sreenivas T, Navale A, Padmanabhan N (2012) Solvent extraction of uranium from lean grade acidic sulfate leach liquor with Alamine 336 reagent. J Radio Nucl Chem 294(1):13–18
Kim C-J, Kumar JR, Kim J-S, Lee J-Y, Yoon H-S (2012) Solvent extraction studies on uranium using amine based extractants and recovery from low grade ore leach liquors. J Braz Chem Soc 23(7):1254–1264
Rydberg J (2004) Solvent extraction principles and practice, revised and expanded edn. CRC Press, Boca Raton
Morais C, Gomiero L, Scassiotti Filho W, Rangel H (2005) Uranium stripping from tertiary amine by sulfuric acid solution and its precipitation as uranium peroxide. Miner Eng 18(13):1331–1333
Hurst F, Crouse D (1961) Recovery of Uranium from Amine Extractants with Ammonium Carbonate. Oak Ridge National Lab, Tenn
Pandey V, Chakraborty A, Maity N (1991) Preparation of nuclear grade uranium oxide from Jaduguda leach liquor. Proceedings of the international symposium on uranium technology [held at Bombay during 13–15 Dec 1989], vol 2. Bhabha Atomic Research Centre, India, p 653
Brown KB, Coleman CF, Crouse DJ, Denis JO, Moore JG (1954) The use of amines as extractants for uranium from acidic sulfate liquors. A preliminary report (No. AECD-4142). Oak Ridge National Lab, Tenn
Crouse D, Brown K (1955) Amine extraction processes for uranium recovery from sulfate liqours, vol I. Oak Ridge National Lab, Tenn
Zahakifar F, Davarkhah R, Charkhi A, Torab-Mostaedi M (2017) Solvent extraction of uranium (VI) from leach liquor solution of Bandar Abbas Gachin ore using Alamine 336. Nucl Sci Technol (in press)
Madaeni S, Jamali Z, Islami N (2011) Highly efficient and selective transport of methylene blue through a bulk liquid membrane containing Cyanex 301 as carrier. Sep Purif Technol 81(2):116–123
Saf AÖ, Alpaydin S, Sirit A (2006) Transport kinetics of chromium (VI) ions through a bulk liquid membrane containing p-tert-butyl calix [4] arene 3-morpholino propyl diamide derivative. J Membr Sci 283(1):448–455
Kogelnig D, Stojanovic A, Jirsa F, Körner W, Krachler R, Keppler BK (2010) Transport and separation of iron (III) from nickel (II) with the ionic liquid trihexyl (tetradecyl) phosphonium chloride. Sep Purif Technol 72(1):56–60
Szczepański P, Diaconu I (2012) Transport of p-nitrophenol through an agitated bulk liquid membrane. Sep Sci Technol 47(12):1725–1732
Yilmaz A, Kaya A, Alpoguz HK, Ersoz M, Yilmaz M (2008) Kinetic analysis of chromium (VI) ions transport through a bulk liquid membrane containing p-tert-butylcalix [4] arene dioxaoctylamide derivative. Sep Purif Technol 59(1):1–8
Ma M, Chen B, Luo X, Tan H, He D, Xie Q, Yao S (2004) Study on the transport selectivity and kinetics of amino acids through di (2-ethylhexyl) phosphoric acid-kerosene bulk liquid membrane. J Membr Sci 234(1):101–109
He D, Ma M, Zhao Z (2000) Transport of cadmium ions through a liquid membrane containing amine extractants as carriers. J Membr Sci 169(1):53–59
Szpakowska M, Nagy OB (2000) Influence of physico-chemical control and of membrane composition on the coupled transport of copper (II) ions through bulk liquid membranes. J Membr Sci 168(1):183–186
León G, Guzmán MA (2005) Kinetic study of the effect of carrier and stripping agent concentrations on the facilitated transport of cobalt through bulk liquid membranes. Desalination 184(1–3):79–87
Altin S, Demircioglu N, Peker I, Altin A (2007) Effects of acceptor phase and donor phase properties on sodium ions transport from aqueous solutions using liquid membrane systems. Colloids Surf A 306(1):14–21
Zaharia I, Aboul-Enein HY, Diaconu I, Ruse E, Bunaciu AA, Nechifor G (2013) Facilitated transport of 5-aminosalicylic acid through bulk liquid membrane. J Iran Chem Soc 10(6):1129–1136
Akin I, Erdemir S, Yilmaz M, Ersoz M (2012) Calix [4] arene derivative bearing imidazole groups as carrier for the transport of palladium by using bulk liquid membrane. J Hazard Mater 223:24–30
Alpoguz HK, Memon S, Ersoz M, Yilmaz M (2002) Transport of Hg 2+ through bulk liquid membrane using a bis-calix [4] arene nitrile derivative as carrier: kinetic analysis. New J Chem 26(4):477–480
Alpoguz HK, Memon S, Ersoz M, Yilmaz M (2002) Transport of metals through a liquid membrane containing calix [4] arene derivatives as carrier. Sep Sci Technol 37(9):2201–2215
Alpoguz HK, Memon S, Ersoz M, Yilmaz M (2003) Kinetics of mercury (II) transport through a bulk liquid membrane containing calix [4] arene derivatives as carrier. Sep Sci Technol 38(7):1649–1664
Muthuraman G, Teng TT, Leh CP, Norli I (2009) Use of bulk liquid membrane for the removal of chromium (VI) from aqueous acidic solution with tri-n-butyl phosphate as a carrier. Desalination 249(2):884–890
Ersoz M (2007) Transport of mercury through liquid membranes containing calixarene carriers. Adv Colloid Interface Sci 134:96–104
Yakubu N, Dudeney A (1987) A study of uranium solvent extraction equilibria with Alamine 336 in kerosene. Hydrometallurgy 18(1):93–104
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zahakifar, F., Charkhi, A., Torab-Mostaedi, M. et al. Kinetic study of uranium transport via a bulk liquid membrane containing Alamine 336 as a carrier. J Radioanal Nucl Chem 316, 247–255 (2018). https://doi.org/10.1007/s10967-018-5739-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-5739-y