Abstract
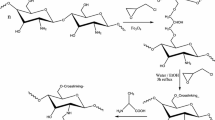
The magnetic chitosan nano-particles functionalized with alanine (or valine) were prepared for enhancing Th(IV) sorption. The uptake kinetics followed the pseudo-second-order model whilst the sorption isotherms fitted to the Langmuir model. The maximum Th(IV) sorption capacities are 128.7 and 154.0 mg/g for MCN-Val and MCN-Ala sorbents, respectively. MCN-Ala has higher Th(IV) sorption capacity since it contains more sorption sites (amino and carboxylic groups) and has less steric hindrance. The values of thermodynamic parameters indicate that Th(IV) sorption is endothermic and spontaneous. The fast kinetics and magnetic properties of the nano-sorbents allow their efficient Th(IV) sorption and magnetic separation from the solution.







Similar content being viewed by others
References
Heuer D, Merle-Lucotte E, Allibert M, Brovchenko M, Ghetta V, Rubiolo P (2014) Towards the thorium fuel cycle with molten salt fast reactors. Ann Nucl Energy 64:421–429
Kaynar ÜH, Şabikoğlu I, Kaynar SC, Eral M (2016) Modeling of thorium (IV) ions adsorption onto a novel adsorbent material silicon dioxide nano-balls using response surface methodology. Appl Radiat Isotopes 115:280–288
Ueno K, Hoshi M (1970) The precipitation of some actinide element complex ions by using hexammine cobalt(III) cation–I: the precipitation of thorium and plutonium(IV) carbonate complex ions with hexammine cobalt(III) chloride. J Inorg Nucl Chem 32:3817–3822
Bayyari M, Nazal M, Khalili F (2010) The effect of ionic strength on the extraction of Thorium(IV) from nitrate solution by didodecylphosphoric acid (HDDPA). J Saudi Chem Soc 14:311–315
Kiliari T, Pashalidis I (2011) Thorium determination in aqueous solutions after separation by ion-exchange and liquid extraction. J Radioanal Nucl Chem 288:753–758
Ansari SA, Mohapatra PK (2017) A review on solid phase extraction of actinides and lanthanides with amide based extractants. J Chromatogr A 1499:1–20
Iida Y, Yamaguchi T, Tanaka T, Hemmi K (2016) Sorption behavior of thorium onto granite and its constituent minerals. J.Nucl. Sci. Technol 10(1):1–12
Lu S, Li H, Zhang F, Du N, Hou W (2016) Sorption of Pb(II) on carboxymethyl chitosan-conjugated magnetite nanoparticles: application of sorbent dosage-dependent isotherms. Colloid Polym Sci 294:1369–1379
Galhoum AA, Mahfouz MG, Gomaa NM, Vincent T, Guibal E (2015) Uranium (VI) sorption using functionalized-chitosan magnetic nanobased particles. Adv Mater Res 1130:499–502
Zhu X, Zhang Z, Yan G (2016) Methylene blue adsorption by novel magnetic chitosan nanoadsorbent. J Water Environ Technol 14:96–105
Tavengwa NT, Cukrowska E, Chimuka L (2016) Modelling of adsorption isotherms and kinetics of uranium sorption by magnetic ion imprinted polymers. Toxico Environ Chem 98:1–12
Chauhan S (2015) Modification of chitosan for sorption of metal ions. J Chem Pharm Res 7:49–55
Wang XL, Yuan LY, Wang YF, Li ZJ, Lan JH, Liu YL (2012) Mesoporous silica sba-15 functionalized with phosphonate and amino groups for uranium uptake. Sci China Chem 55:1705
Atia AA (2005) Studies on the interaction of mercury(II) and uranyl(II) with modified chitosan resins. Hydrometallurgy 80:13–22
Oshita K, Sabarudin A, Takayanagi T, Oshima M, Motomizu S (2009) Adsorption behavior of uranium(VI) and other ionic species on cross-linked chitosan resins modified with chelating moieties. Talanta 79:1031–1035
Choi SH, Nho Y (2000) Adsorption of UO2 2+ by polyethylene adsorbents with amidoxime, carboxylic, and amidoxime/carboxylic group. Radiat Phys Chem 57:187–193
Wang JS, Peng RT, Yang JH, Liu YC, Hu XJ (2011) Preparation of ethylenediamine-modified magnetic chitosan complex for adsorption of uranyl ions. Carbohydr Polym 84:1169–1175
Galhoum AA, Mahfouz MG, Atia AA, Abdel-Rehem ST, Gomaa NA, Vincent T, Guibal E (2015) Amino acid functionalized chitosan magnetic nanobased particles for uranyl sorption. Ind Eng Chem Res 54:12374–12385
Galhoum AA, Mahfouz MG, Gomaa NM, Vincent T, Guibal E (2017) Chemical modifications of chitosan nano-based magnetic particles for enhanced uranyl sorption. Hydrometallurgy 168:127–134
Rohwer H, Rheeder N, Hosten E (1997) Interactions of uranium and thorium with Arsenazo III in an aqueous medium. Anal Chim Acta 341:263–268
Han YC, Cha HG, Chang WK, Kang YS (2007) Synthesis of highly magnetized iron nanoparticles by a solventless thermal decomposition method. J Phys Chem C 111:6275–6280
Mahfouz MG, Galhoum AA, Gomaa NA, Abdel-Rehem SS, Atia AA, Vincent T, Guibal E (2015) Uranium extraction using magnetic nano-based particles of diethylenetriamino -functionalized chitosan: equilibrium and kinetic studies. Chem Eng J 262:198–209
Hosseini S, Ibrahim F, Djordjevic I, Koole LH (2014) Polymethyl methacrylate-co- methacrylic acid coatings with controllable concentration of surface carboxyl groups: a novel approach in fabrication of polymeric platforms for potential bio-diagnostic devices. Appl Surf Sci 300:43–50
Philip XR, Michelini M, Gibson JK (2013) Proton transfer in Th(IV) hydrate clusters: a link to hydrolysis of Th(OH) 2+2 to Th(OH) +3 in aqueous solution. J Phys Chem A 117:451–459
Talip Z, Eral M, Hiçsönmez Ü (2009) Adsorption of thorium from aqueous solutions by perlite. J Environ Radioact 100:139–143
Xu J, Zhou L, Jia Y, Liu Z, Adesina AA (2015) Adsorption of thorium (iv) ions from aqueous solution by magnetic chitosan resins modified with triethylene-tetramine. J Radioanal Nucl Chem 303:347–356
Wang X, Fan Q, Yu S, Chen Z, Ai Y, Sun Y (2016) High sorption of U(VI) on graphene oxides studied by batch experimental and theoretical calculations. Chem Eng J 287:448–455
He F, Wang H, Wang Y, Wang X, Zhang H, Li H, Tang J (2013) Magnetic Th(IV)-ion imprinted polymers with salophen Schiff base for separation and recognition of Th(IV). J Radioanal Nucl Chem 295:167–177
He Q, Chang X, Wu Q, Huang X, Hu Z, Zhai Z (2007) Synthesis and applications of surface-grafted Th(IV)-imprinted polymers for selective solid-phase extraction of thorium(IV). Anal Chim Acta 605:192–197
Birlik E, Buyuktiryaki S, Ersoz A, Say R, Denizli A (2006) Selective separation of thorium using ion imprinted chitosanphthalate particles via solid phase extraction. Sep Sci Technol 41:3109–3121
Wang H (2011) Selective preconcentration of trace thorium from aqueous solutions with Th(IV)-imprinted polymers prepared by a surface-grafted technique. Int J Environ Anal Chem 91:1050–1061
Akkaya R, Ulusoy U (2008) Adsorptive features of chitosan entrapped in polyacrylamide hydrogel for Pb2+, UO2 2+, and Th4+. J Hazard Mater 151:380–388
Humelnicu D, Dinu M, Dragan E (2011) Adsorption characteristics of UO2 2+ and Th4+ ions from simulated radioactive solutions onto chitosan/clinoptilolite sorbents. J Hazard Mater 185:447–455
Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation (21366001; 21667001), the International Scientific and Technological Cooperation Projects (2015DFR61020), the Key Research and Development Program and the Natural Science Fund Program of Jiangxi Province (20161BBF60059; S2017ZRMSB0473).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, T., Chen, Q., Zhou, L. et al. Efficient sorption of Th(IV) from aqueous solutions onto magnetic chitosan nano-particles functionalized with alanine and valine. J Radioanal Nucl Chem 314, 1083–1093 (2017). https://doi.org/10.1007/s10967-017-5478-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5478-5