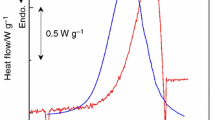
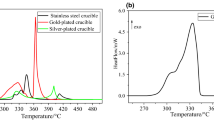
Abstract
The thermal behavior and kinetic analysis of ammonium uranyl carbonate decomposition has been studied in inert gas, O2, and 90%Ar–10%H2 atmospheres under non-isothermal conditions. The results showed a dependence on specific surface area with the decomposition temperature of ammonium uranyl tri-carbonate (AUC). Specific surface area increases and reaches a maximum between 300 and 400 °C and decreases at T > 400 °C. The reaction paths of AUC decomposition under the three atmospheres were proposed. The integral methods Flynn–Wall–Ozawa (FWO) and Kissinger–Akahira–Sunose (KAS) were used for the kinetic analysis. The activation energy averages are 58.01 and 56.19 kJ/mol by KAS and FWO methods, respectively.











Similar content being viewed by others
References
Slycke J, Mittemeijer EJ, Somers MAJ (2015) Thermodynamics and kinetics of gas and gas–solid reactions. In: Mittemeijer EJ, Somers MAJ (eds) Thermochemical surface engineering of steels. Elsevier-Woodhead, Amsterdam, pp 3–111
Vyazovkin S, Chrissafis K, Di Lorenzo ML, Koga N, Pijolat M, Roduit B, Sbirrazzuoli N, Suñol JJ (2014) ICTAC kinetics committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta 590:1–23
Khawam A, Flanagan DR (2006) Solid-state kinetic models: basics and mathematical fundamentals. J Phys Chem B 110:17315–17328
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N (2011) ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19
Flynn JH (1983) The iso-conversional method for determination of energy ofactivation at constant heating rates corrections for the Doyle approximation. J Therm Anal 27:95–102
Draper LA, Sveum KL (1970) The analysis of thermal data. Thermochim Acta 1:345–365
Klemm U, Sobek D (1989) Influence of admixing of lubricants on compressibility and compactibility of uranium dioxide powders. Powder Technol 57(2):135–142
Wang Y, Chen Q, Shen X (2016) Preparation of low-temperature sintered O2 nanomaterials by radiolytic reduction of ammonium uranyl tricarbonate. J Nucl Mater 479:162–166
Ganguly C (2001) Nuclear reactor fuel fabrication (including quality control), 2nd edn. Encyclopedia of Materials: Science and Technology, pp 1–15
Marajofsky A, Perez L, Celora J (1991) On the dependence of characteristics of powders on the AUC process parameter. J Nucl Mater 178:43–151
Pijolat M, Brun C, Valdivieso F, Soustelle M (1997) Reduction of uranium oxide U3O8 to UO2 by hydrogen. Solid State Ionics 101–103:931–935
Tel H, Eral M (1996) Investigation of production conditions and powder properties of AUC. J Nucl Mater 231:165–169
Dahale ND, Chawla KL, Jayadevan NC, Venugopal V (1997) X-ray, thermal and infrared spectroscopic studies on lithium and sodium oxalate hydrates. Thermochim Acta 293:163–166
Li Y, Penga J, Liua B, Li W, Huang D, Zhang L (2011) Prediction model of ammonium uranyl carbonate calcination by microwave heating using incremental improved back-propagation neural network. Nucl Eng Des 241:1909–1913
Dollimore D, Clough P (1985) A study of the thermal decomposition of uranyl acetate using infra-red spectroscopic techniques. Thermochim Acta 85:43–46
Bachmann HG, Seibold K, Dokuzoguz HZ, Muller HM (1975) X-ray powder diffraction and some thermodynamic data for (NH4)4[UO2(CO3)3]. J Inorg Nucl Chem 37:735–737
Haaldahl L, Sorensen T (1979) Thermal analysis of the decomposition of ammonium uranyl carbonate (AUC) in different atmospheres. Thermochim Acta 29:253–259
Haaldahl L, Nygren M (1984) TG, DSC, X ray and electron diffraction studies of intermediate phases in the reduction of ammonium uranyl carbonate to O2. Thermochim Acta 72:213–218
Haaldahl L (1984) In situ studies of the decomposition of ammonium uranyl carbonate in an electron microscope. J Nucl Mater 126:170–176
Baran V, Voseček V (1987) Spontaneous isothermal decomposition of the uranyl carbonate complex (NH4)4[UO2(CO3)3] at room temperature: part II. Infrared spectroscopy and X-ray analysis. Thermochim Acta 122(2):261–276
Bing-Guo L, Jin-Hui P, Srinivasakannan C, Li-Bo Z, Jin-Ming H, Sheng-Hui G, Dong-Cheng K (2015) Preparation of U3O8 by calcination from ammonium uranyl carbonate in microwave fields: process optimization. Ann Nucl Energy 85:879–884
Wang YM, Chen QD, Shen XH (2017) One-step synthesis of hollow UO2 nanospheres via radiolytic reduction of ammonium uranyl tricarbonate. Chin Chem Lett 28:3–18
Haaldahl L, Nygren M (1985) A study of the composition of the amorphous phase formed during decomposition of ammonium uranyl carbonate in various atmospheres. Thermochim Acta 95:389–994
Haaldahl L, Nygren M (1986) Thermal analysis studies of the reactions occurring during the decomposition of ammonium uranyl carbonate in different atmospheres. J Nucl Mater 138:99–106
Asadi Z, Ranjkesh Shorkaei M (2013) Synthesis, X-ray crystallography, thermal studies, spectroscopic and electrochemistry investigations of uranyl Schiff base complexes. Spectrochim Acta Part A 105:344–351
Kianfar AH, Dostani M (2011) Synthesis, spectroscopy, and thermal study of uranyl unsymmetrical Schiff base complexes. Spectrochim Acta Part A 82:69–73
Savchenkov AV, Peresypkina EV, Pushkin DV, Virovets AV, Serezhkina LB, Serezhkin VN (2014) Structural features of two polymorphs of ammonium uranyl crotonate. J Mol Struct 1074:583–588
Kim KW, Lee KY, Chung DY, Lee EH, Moon JK, Shin DW (2012) Evaluation of the stability of uranyl peroxo-carbonato complex ions in carbonate media at different temperatures. J Hazard Mater 233–234:213–218
Notz KJ, Haas PA (1989) Properties and thermal decomposition of the double salts of uranyl nitrate-ammonium nitrate. Thermochim Acta 155:283–295
de Aquino AR, Isolani PC, Zukerman-Schpector J, Zinner LB, Vicentini G (2001) Uranyl nitrate complexes with lactams. J Alloy Compd 323–324:18–21
Alvarenga MG, Zinner LB, Fantin CA, Matos JR, Vicentini G (2004) Preparation and characterization of uranyl complexes with three isomeric methyl-pyridine-N-oxide ligands. J Alloy Compd 374:258–260
Sreenivasan NL, Srinivasan TG, Vasudeva Rao PR (1994) A spectrophotometric method for the determination of the oxygen to metal ratio in U3O8. Radioanal Nucl Chem Lett 188(6):463–470
Hung NT, Thuan LB, Khoai DV, Lee JY, Kumar JR (2016) Brandon mathematical model describing the effect of calcination and reduction parameters on specific surface area of UO2 powders. J Nucl Mater 474:150–154
Girgis BS, Rofail NH (1992) Decomposition-reduction stages of ammonium uranyl carbonates under different atmospheres. Thermochim Acta 196:105–115
Kim EH, Park JJ, Chang JH, Choi CS, Kim SD (1994) Thermal decomposition kinetics of ammonium uranyl carbonate. J Nucl Mater 209:294–300
Qingren GE, Shifang K (1987) Study of AUC thermal decomposition kinetics in kinetics in nitrogen by a non-isothermal method. Thermochim Acta 116:71–77
Kim BH, Lee YB, Prelas MA, Ghosh TK (2012) Thermal and X-ray diffraction analysis studiesduring the decomposition of ammonium uranyl nitrate. J Radioanal Nucl Chem 292:1075–1083
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Korichi, S., Mernache, F., Benaouicha, F. et al. Thermal behavior and kinetic modeling of (NH4)4UO2(CO3)3 decomposition under non-isothermal conditions. J Radioanal Nucl Chem 314, 923–934 (2017). https://doi.org/10.1007/s10967-017-5444-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5444-2