Abstract
Liquid scintillation spectrometry is widely used for the analysis of alpha and beta emitting radionuclides. Robust calibration of liquid scintillation (LS) spectrometers is fundamental to accurate LS measurement but at the same time is time consuming and costly, particularly if a wide range of radionuclides are analysed by the laboratory. The frequency of the calibration varies in different laboratories and is based on many practical and operational factors. This work summarizes the observations regarding variations in 1220 Quantulus spectrometers efficiency calibrations performed annually using various radionuclides: 3H 63Ni, 55Fe, 36Cl, 45Ca, 147Pm, 241Pu, 99Tc for a period of 9 years and discusses the implication to calibration frequency.
Similar content being viewed by others
Introduction
Liquid scintillation (LS) spectrometry has been a very important tool in radiation measurement field since mid-twentieth century when first attempts to measure 14C by this method were made [1]. The technique has developed over years and nowadays the liquid scintillation spectrometers can be found in many institutions related to a wide area of applications: from nuclear industry and safety, through radiopharmaceutical industry to a number of research-related organizations (universities, research institutes). Modern LS counters offer the possibility of both qualitative and quantitative analysis of alpha and beta emitters with high precision and accuracy, offering at the same time low detection limits. As many other instrumental techniques, liquid scintillation spectrometry requires robust and reliable calibration to accurately measure radiation [2, 3]. However, the detection efficiency of the counting equipment will vary depending on a range of factors including radionuclide emission and associated energy, sample composition, scintillator and vial type. All these factors must be accurately quantified and corrected for during routine measurement. In addition, the detection efficiency will vary with time due to deterioration of instrument optics and periodic recalibration is therefore required. In order to address these issues the most commonly used approach is to calibrate the LS spectrometers using a set of standards of known activity and a varying amount of quench.
Full calibration of a liquid scintillation counter is time consuming, particularly if a wide range of radionuclides are analysed by the laboratory. It can also be expensive if frequent purchase of new certified standard is required e.g., for radionuclides with short half-live in a range of days or weeks. Use of high energy beta emitters (e.g. 90Sr/90Y or 32P) may also carry an additional radiological risk.
The frequency at which full calibrations are performed will depend on the laboratory and is based on a range of practical and operational factors. However, to inform this choice, it is useful to understand the long term stability of liquid scintillation counters and the rate of degradation of the system. In addition, knowledge of the long term stability of the LS calibration can be used in assessing method uncertainties associated with calibration. This work presents the summary of the Quantulus LSC calibrations performed annually using various radionuclides: 63Ni, 55Fe, 36Cl, 45Ca, 147Pm, 241Pu, 99Tc for a period of 10 years and assesses the long term stability of the instruments.
Experimental
A set of calibration equations for three different 1220 Quantulus counters (Quantulus 1, Quantulus 2 and Quantulus 3), obtained between years 2007 and 2016, was used. Radionuclides considered are presented in Table 1. Counting efficiency calibrations for all the radionuclides were prepared using certified standard solutions traceable to National Physical Laboratory (UK), National Institute for Standard and Technology (USA) or Physikalish-Technische Bunsesanstaldt (Germany). The different quench level for any given set of calibration standards was achieved by the addition of varying amounts of a quenching agent (e.g. nitromethane) or by using different sample solution to scintillation cocktail ratios. As the quench level estimation method the SQPE (external standard quench parameter) approach was used [4]. This method is based on the comparison of two spectra: first one is obtained for the sample exposed to the gamma radiation from the external source, second one is obtained for the sample alone. Both spectra are collected for the same amount of time and the non-exposed spectrum is subtracted from the exposed one. The channel number where 99% of the net spectrum counts lay below is used as the measure of quench in the analysed sample. The details on calibration standards preparation were combined in Table 2. The deviations in counting efficiencies were checked for selected SQPE values representing varying degree of quench; from heavily quenched samples (SQPE 1), through a mid-level (SQPE 2) to relatively weakly affected ones (SQPE 3). The SQPE values used for the comparison purposes were fixed for any given radionuclide and the calibration date and are presented in Table 3 together with a typical calibration curve fit uncertainty (2σ) observed in the considered timescale. Each calibration curve fit (2nd degree polynomial) was used to calculate counting efficiencies for each radionuclide at different SQPE value. The obtained values were plotted against calibrtation date.
Results and discussion
The obtained results for variation in counting efficiency between years 2007 and 2016 were plotted for each radionuclide, spectrometer and quench level. A linear trendline was fitted to each set of data—the slope parameter of the obtained fit equation represents a rate of annual change in the absolute counting efficiency. For each individual spectrometer and quench level an average annual absolute counting efficiency change was calculated. These numbers were further averaged to produce a single value representing an annual change in absolute counting efficiency for all three spectrometers. The summary of the obtained results for 3H, 241Pu, 55Fe, 63Ni, 147Pm, 45Ca, 99Tc and 36Cl is presented in Tables 4, 5, 6, 7, 8, 9, 10, 11 and 12, respectively. For 147Pm two separate tables (Tables 8, 9) are presented to reflect the change in counting window modification from 1–600 channels to 301–650 channels. The counting window modification was introduced to allow the elimination of potential of 151Sm (β max = 76.3 keV) interference with 147Pm measurement as both elements show similar chemical properties and the complete isolation of the two radionuclides is challenging.
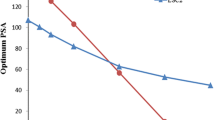
Plots for the observed counting efficiency change in time for Quantulus 1 and medium quench level, typical for most of the analysed samples, are presented in Fig. 1.
Counting efficiency change plots for Quantulus 1 at medium quench level (SQPE 2). Gaps in data series are caused by lack of data (no calibration because of standard unavailability). Step change in 147Pm in 2014 counting efficiency is the result of modification to the counting window selected for the analysis
Absolute counting efficiency for low energy beta emitters (3H, 241Pu) and 55Fe drops by approximately 0.5–1% every year. Similar observation for 3H was observed previously [2]. Such relatively high degradation rate of the counting efficiency may be explained by slow deterioration of the counter optics caused by the slow release of vapours (both acidic and basic) from the counted sources over time and progressive dust particles accumulation. Since the light originating from low-energy beta emitters is sensitive to any disturbance in the optics of the instrument due to its lower intensity, taking a greater care on the instruments condition (e.g., annual cleanup) should result in reduced counting efficiency deterioration. A step change in counting efficiency visible for 55Fe in year 2012 was caused by the use of a new standard solution. This solution was used for the calibration purposes only in year 2012 and was replaced in the following years by other 55Fe standard solutions.
Radionuclides emitting more energetic beta radiation show much greater counting efficiency stability over long time periods which was previously observed for 14C [5]. During current checks a slight positive change can be observed for 147Pm, 99Tc and 36Cl. As an increase of the spectrometer performance in time is very unlikely, the effect can most probably be explained by the slow evaporative loss and associated concentration of the stock solutions used to prepare the calibration standards. Typical initial mass of the freshly prepared stock solution is approx. 30 g. Activity concentration change of 0.2% every year (as suggested by 99Tc counting efficiency increase) leads to activity change of ca. 2% over the study period of nine years. This corresponds to approximately mass change of 0.6 g over nine years or, on average, 0.07 g/year. Because the standard bottle is usually opened many times every year to produce calibration standards, test sources, quality control solutions, etc., it is likely that the standard solution evaporation is the cause of the observed positive counting efficiency change for some radionuclides. Similar analysis could be done for 147Pm and 36Cl standards where the positive counting efficiency change trend of approximately 0.3%/year can be observed, leading to an annual average solution mass losses of 0.09 g.
Such a hypothesis may be further strengthened by the fact that all the calibration standards used for these three radionuclides can be traced back to a single original stock solution bottle stored for several years in a PFA bottle at ambient temperature. Further, the analysis of 45Ca behavior shows a decrease in counting efficiency similar to low-energy beta emitters, ca. 0.4% every year. In this case the calibration standards used to calibrate the spectrometers can be traced back to different original certified standards—mainly because of a short 45Ca half-live preventing the use of one stock solution over a long timescale.
Taking into account the fact that for the low-energy beta emitters, where counting efficiency is typically 20–30%, the absolute counting efficiency change by 1% every year translates to a relative counting efficiency change between 3 and 5%, care must be taken to observe the trend and update the counting efficiency accordingly on more frequent basis or to increase the total method uncertainty to allow for such a rapid change [6]. For high energy beta emitters, where typical counting efficiencies are in the range of 80–100% the effect is much less significant and corresponds to relative counting efficiency change at a level of 1%. Of course such changes, however small, should also be taken into account while constructing the expanded uncertainty budget.
One of possible conclusions from the calibration stability comparison for different radionuclides is that for an instrument with “undisturbed” (not cleaned or heavily serviced) optics/photomultiplier tubes windows, the counting efficiency drops at a steady rate between 0.5 and 1% every year. This degradation rate can be observed over a fairly broad range of beta emission energies of the considered radionuclides. The more stable counting efficiency behaviour for high-energy beta emitters may be a genuine stability as well as an apparent effect caused by the dropping counter efficiency balanced by the slow activity concentration increase of the primary standard solutions used to prepare the calibration standards.
Conclusions
The degree of the observed changes in counting efficiency is similar for all three instruments used in this comparison which suggests that the degradation of the electronic and/or optical components of each system takes place at similar pace. These similarities seem to be linked to the external environment (instrument location) and chemical form of the samples being counted as the most important factors influencing the long-term stability of the counters. The reliability of the radioactive standard solutions over long timescale seems to be another factor which could influence the calibration of the spectrometers, as evaporation, adsorption on the container walls or any other not yet identified process may lead to alteration of the specific activity of a standard solution which in turn causes errors in any calculations which are based on the activity measurements of such solutions.
Depending on particular analytical needs, it is possible to extend the calibration periods for medium and high-energy beta emitters. For low-energy beta emitters the calibration frequency should depend on the observation of the trend in counting efficiency change. In both cases the rate of counting efficiency change should be built into the overall measurement uncertainty budget.
References
Annunziata MF, Kessler MJ (2012) In: Annunziata MF (ed) Handbook of radiochemical analysis, 3rd edn. Academic Press, Oxford
Use and preparation of quench curves in LSC. http://www.perkinelmer.com/liquidscintillation/images/APP_Use-and-Preparation-of-Quench-Curves-in-LSC_tcm151-171749.pdf. Accessed 26 May 2017
Pochwalski K, Broda R, Radoszewski T (1988) Standardization of pure beta emitters by liquid scintillation counting. Appl Radiat Isot 39:165–172
Minne E, Heynen F, Hallez S (2008) Possible overestimation of the external standard quench parameter on Wallac 1220 Quantulus™ with high energetic beta-emitters. J Radioanal Nucl Chem 278:39–45
Jaubert F, Tartés I, Cassette P (2006) Quality control of liquid scintillation counters. Appl Radiat Isot 64:1163–1170
Rusconi R, Forte M, Caresana M, Bellinzona S, Cazzaniga MT, Sgorbati G (2006) The evaluation of uncertainty in low-level LSC measurements of water samples. Appl Radiat Isot 64:1124–1129
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gaca, P., Warwick, P.E. & Croudace, I.W. Liquid scintillation counters calibration stability over long timescales. J Radioanal Nucl Chem 314, 753–760 (2017). https://doi.org/10.1007/s10967-017-5388-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5388-6