Abstract
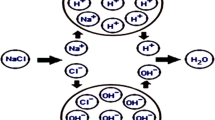
In water treatment by ionizing radiation ·OH is suggested to initiate the degradation of organics. In these reactions mostly carbon centred radicals form. Here we show that other inorganic radicals also highly contribute to the initiation of degradation. Cl− and HCO3 − in the treated water reacting with ·OH transform to Cl ·−2 and CO ·−3 . In their reactions C-centred radicals form, too. The reactions of e −aq and H· water radiolysis intermediates may also produce carbon centred radicals. The C-centred radicals react readily with dissolved O2, this is the starting step of the gradual oxidation.
Similar content being viewed by others
References
Getoff N (1997) Peroxyl radicals in the treatment of waste solutions. In: Alfassi ZB (ed) Peroxyl radicals. Wiley, New York, pp 483–506
Stefan M (2016) Advanced oxidation processes for water and wastewater treatment. IWA Publishing, London
IAEA (2007) Radiation processing, environmental applications. International Atomic Energy Agency, Vienna
Wojnárovits L, Takács E, Szabó L (2016) Gamma-ray and electron beam-based AOPs. In: Stefan M (ed) Advanced oxidation processes for water and wastewater treatment, vol 6. IWA Publishing, London
Woods RJ, Pikaev AK (1994) Applied radiation chemistry: radiation processing. Wiley, New York
Wardman P (1989) Reduction potentials of one-electron couples involving free radicals in aqueous solution. J Phys Chem Ref Data 18:1637–1755
Mostofa KMG, Liu C-Q, Mottaleb MA, Wan G, Ogawa H, Vione D, Yoshioka T, Wu F (2013) Dissolved organic matter in natural waters. In: Mostofa KMG, Yoshioka T, Mottaleb MA, Vione D (eds) Photobiogeochemistry of organic matter. Environmental science and engineering. Springer, Berlin, pp 1–137
Westerhoff P, Mezyk SP, Cooper WJ, Minakata D (2007) Electron pulse radiolysis determination of hydroxyl radical rate constants with Suwannee River fulvic acid and other dissolved organic matter isolates. Environ Sci Technol 41:4640–4646
Wojnárovits L, Takács E (2013) Structure dependence of the rate coefficients of hydroxyl radical + aromatic molecule reaction. Radiat Phys Chem 87:82–87
Swallow AJ (1973) Radiation chemistry: an introduction. Longman, London
Rickman KA, Mezyk SP (2010) Kinetics and mechanism of sulfate radical oxidation of β-lactam antibiotics in water. Chemosphere 81:359–365
Kazmierczak L, Szala-Bilnik J, Wolszczak M, Swiatla-Wojcik D (2015) Temperature dependence of rate constants for hydrogen atom reaction with Cl ·−2 in water by pulse radiolysis of aqueous HCl solution. Radiat Phys Chem 117:7–11
Buxton G, Greenstock CL, Helman, WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in aqueous solution. J Phys Chem Ref Data 17:513–886. http://kinetics.nist.gov/solution/. Accessed 13 Feb 2016
Neta P, Hiue RE, Ross AB (1988) Rate constants of inorganic radicals in aqueous solution. J Phys Chem Ref Data 17:1027–1284
Madden KP, Mezyk SP (2011) Critical review of aqueous solution reaction rate constants for hydrogen atoms. J Phys Chem Ref Data 40:1–43
Lutze H (2013) Sulfate radical based oxidation in water treatment. Dissertation Dr. rer. nat. Duisburg-Essen. https://duepublico.uni-duisburg-essen.de/servlets/DerivateServlet/Derivate-35021/Lutze_Diss.pdf. Accessed 13 Feb 2016
Criquet J, Leitner NKV (2015) Reaction pathway of the degradation of the p-hydroxybenzoic acid by sulfate radical generated by ionizing radiations. Radiat Phys Chem 106:307–314
von Sonntag C (2006) Free-radical-induced DNA damage and its repair. A chemical perspective. Springer, Heidelberg
Steenken S (1987) Addition-elimination paths in electron-transfer reactions between radicals and molecules. Oxidation of organic molecules by OH radical. J Chem Soc Faraday Trans 1(83):113–124
Ziajka J, Pasiuk-Bronikowska W (2005) Rate constants for atmospheric trace organics scavenging SO ·−4 in the Fe-catalysed autoxidation of S(IV). Atmos Environ 39:1431–1438
Choure SC, Bamatraf MMM, Rao BSM, Das R, Mohan H, Mittal JP (1997) Hydroxylation of chlorotoluenes and cresols: a pulse radiolysis, laser flash photolysis, and product analysis study. J Phys Chem A 101:9837–9845
Roder M, Földiák G, Wojnárovits L (1999) Electron transfer from cresols to N ·3 , BrO ·2 , ClO ·2 , NO ·2 and SO ·−4 radicals: correlation between rate constants and one-electron reduction potentials. Radiat Phys Chem 55:515–519
Neta P, Madhavan V, Zemel H, Fessenden RW (1977) Rate constants and mechanism of reaction of SO ·−4 with aromatic compounds. J Am Chem Soc 99:163–164
Canle Lopez M, Rodríguez S, Rodríguez Vazques LF, Santaballa JA, Steenken S (2001) First stages of photodegradation of the urea herbicides Fenuron, Monuron and Diuron. J Mol Struct 565–566:133–139
Canle Lopez M, Fernandez MI, Rodríguez S, Santaballa JA, Steenken S, Vulliet E (2005) Mechanisms of direct and TiO2-photocatalised UV degradation of phenylurea herbicides. Chem Phys Phys Chem 6:2064–2074
Asmus KD (1979) Stabilization of oxidized sulfur centers in organic sulfides. Radical cations and odd-electron sulfur-sulfur bonds. Acc Chem Res 12:436–442
Szabó L, Tóth T, Takács E, Wojnárovits L (2016) One-electron oxidation of molecules with aromatic and thioether functions: Cl ·−2 /Br ·−2 and OH induced oxidation of penicillins studied by pulse radiolysis. J Photochem Photobiol 326:50–59
Hasegawa K, Neta P (1978) Rate constants and mechanisms of reaction of Cl ·−2 . J Phys Chem 82:854–857
O’Neil P, Steenken S, Schulte-Frohlinde P (1975) Formation of radical cations of methoxylated benzenes by reaction with OH radicals, Tl2+, Ag2+ and SO ·−4 in aqueous solution. An optical and conductometric pulse radiolysis and in situ radiolysis electron spin resonance study. J Phys Chem 79:2773–2779
Paul J, Naik DB, Bhardwaj YK, Varshney L (2014) Studies on oxidative radiolysis of ibuprofen in presence of potassium persulfate. Radiat Phys Chem 100:38–44
Bíró Á, Takács E, Wojnárovits L (1996) Rate constants for the reaction of hydrated electrons and hydroxyl radicals with acrylate monomers. Macromol Rapid Commun 17:353–357
Elliot AJ, McCracken DR, Buxton GV, Wood ND (1990) Estimation of rate constants for near-diffusion-controlled reactions in water at high temperatures. J Chem Soc Faraday Trans 86:1539–1547
Ashton L, Buxton GV, Stuart CR (1995) Temperature dependence of the rate of reaction of OH with some aromatic compounds in aqueous solution. Evidence for the formation of a π-complex intermediate? J Chem Soc Faraday Trans 91:1631–1633
Wojnárovits L, Takács E (2014) Rate coefficients of hydroxyl radical reactions with pesticide molecules and related compounds: a review. Radiat Phys Chem 96:120–134
Albarran G, Schuler RH (2005) Concerted effects of substituents in the reaction of OH radicals with aromatics: the cresols. J Phys Chem A 109:9363–9370
Albarran G, Schuler RH (2007) Hydroxyl radical as a probe of the charge distribution in aromatics: phenol. J Phys Chem A 111:2507–2510
Albarran G, Mendoza E, Schuler RH (2016) Concerted effects of substituents in the reaction of ·OH radicals with aromatics: the hydroxybenzaldehydes. Radiat Phys Chem 124:46–51
Schuler RH, Albarran G (2002) The rate constants for reaction of OH radicals with benzene and toluene. Radiat Phys Chem 64:189–195
Land EJ, Ebert M (1967) Pulse radiolysis studies of aqueous phenol. Water elimination from dihydroxycyclohexadienyl radicals to form phenoxyl. Trans Faraday Soc 63:1181–1190
Wojnárovits L, Földiák G, D’Angelantonio M, Emmi SS (2002) Mechanism of OH radical-induced oxidation of p-cresol to p-methylphenoxyl radical. Res Chem Intermediat 28:373–386
Chen S-N, Hoffman M, Parsons GH Jr (1975) Reactivity of the carbonate radical toward aromatic compounds in aqueous solution. J Phys Chem 79:1911–1912
Moore JS, Phillips GO, Sosnowski A (1977) Reaction of the carbonate radical anion with substituted phenols. Int J Radiat Biol Relat Stud Phys Chem Med 31:603–605
Huie RE, Shoute LCT, Neta P (1991) Temperature dependence of the rate constants for reactions of the carbonate radical with organic and inorganic reductants. Int J Chem Kinet 23:541–552
Augusto O, Bonini MG, Amanso AM, Linares E, Santos CCX, De Menezes SL (2002) Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radical Biol Med 32:841–859
Chen SN, Hoffman MZ (1973) Rate constants for the reaction of the carbonate radical with compounds of biochemical interest in neutral aqueous solution. Radiat Res 56:40–47
Huang J (2000) Carbonate radical in natural waters, PhD thesis, University of Toronto, Toronto. https://tspace.library.utoronto.ca/bitstream/1807/14631/1/NQ50045.pdf. Accessed 13 Feb 2016
Steenken S, O’Neill P, Schulte-Frohlinde D (1977) Formation of radical zwitterions from methoxylated benzoic acids. 1. One electron oxidation by TI2+, Ag2+, and SO ·−4 . J Phys Chem 81:26–30
Sharma SB, Mudaliar M, Rao BSM, Mohan H, Mittal JP (1997) Radiation chemical oxidation of benzaldehyde, acetophenone and benzophenone. J Phys Chem A 101:8402–8408
Jacobi H-W, Wicktor F, Herrmann H, Zellner R (1999) A laser flash photolysis kinetic study of reactions of the Cl2 − radical anion with oxygenated hydrocarbons in aqueous solution. Int J Chem Kinet 31:159–181
Clifton CL, Huie RE (1993) Rate constants for some hydrogen abstraction reactions of the carbonate radical. Int J Chem Kinet 25:199–203
Buxton GV (2008) An overview of the radiation chemistry of liquids. In: Spotheim-Maurizot M, Mostafavi M, Douki T, Belloni J (eds) Radiation chemistry: From basics to applications in material and life sciences. EDP Sciences, Les Ulis, pp 3–16
Bielski BHJ, Cabelli DE, Arudi RL, Ross AB (1985) Reactivity of HO2/O2 − radicals in aqueous solution. J Phys Chem Ref Data 14:1041–1100
Cooper WJ, Cramer CJ, Martin NH, Mezyk SP, O’Shea KE, von Sonntag C (2009) Free radical mechanism for the treatment of methyl tert-butsyl ether (MTBE) via advanced oxidation/reductive processes in aqueous solutions. Chem Rev 109:1302–1345
Marchaj A, Kelley DG, Bakac A, Espenson JH (1991) Kinetics of the reactions between alkyl radicals and molecular oxygen in aqueous solution. J Phys Chem 95:4440–4441
Rabani J, Klug-Roth D, Henglein A (1974) Pulse radiolytic investigations of OHCH2O2 radicals. J Phys Chem 78:2089–2093
Adams GE, Willson RL (1969) Pulse radiolysis studies on the oxidation of organic radicals in aqueous solution. Trans Faraday Soc 65:2981–2987
Fang X, Pan X, Rahmann A, Schuchmann H-P, von Sonntag C (1995) Reversibility in the reaction of cyclohexadienyl radicals with oxygen in aqueous solution. Chem Eur J 1:423–429
von Sonntag C, Schuchmann H-P (1997) Peroxyl radicals in aqueous solution. In: Alfassi ZB (ed) Peroxyl radicals. Wiley, New York, pp 173–274
von Sonntag C, Schuchmann H-P (2001) The chemistry behind the application of ionizing radiation in water-pollution abatement. In: Jonah CD, Rao BSM (eds) Radiation chemistry: present status and future trends. Studies in physical and theoretical chemistry 87. Elsevier, Amsterdam, pp 657–670
Kim Y, Kim J, Han B (2011) Application of an electron accelerator for the treatment of wastewater from textile dyeing industries. J Korean Phys Soc 59:3489–3493
Acknowledgments
The authors thank Hungarian Science Foundation (OTKA, NK 105802) and International Atomic Energy Agency (Contract No. 16485) for support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wojnárovits, L., Takács, E. Wastewater treatment with ionizing radiation. J Radioanal Nucl Chem 311, 973–981 (2017). https://doi.org/10.1007/s10967-016-4869-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-4869-3