Abstract
The purpose of this study is to synthesize 123I-labeled hesperetin and to investigate its in vivo behavior. The optimized labeling condition provided two isomers of 123I-labeled hesperetin with high radiochemical yields and radiochemical purities. Both 123I-labeled products were orally administered to normal ICR mice, and the initial result showed that most of 123I activity was detected in the stomach and the intestines. A part of 123I-labeled hesperetin was absorbed from the small intestine to bloodstream and then it was distributed in normal organs. The results in the present study provided an efficient radiolabeling method of flavonoid and quantitative organ distribution of orally administered hesperetin.
Similar content being viewed by others
Introduction
The flavanone hesperetin and hesperidin are widely found in a large number of Citrus fruits including sweet orange, mandarin, lemon, and lime [1]. Especially the composition of hesperetin can reach more than 40 % on a dry weight basis of a few immature fruits and high concentrations of hesperetin were also found in orange and grapefruit peel [2]. Hesperetin showed a widely range of pharmacological effects such as anti-inflammatory, antioxidant, anticancer, antiviral, antiallergic, and neuroprotective [3, 4]. Moreover it have been reported to provide a reduced risk of osteoporosis and oral administrations of hesperetin in hypercholesterolemic rat and hamster showed a profound effect for reducing plasma cholesterol and triglycerol levels [5]. In addition to several bioactivities, hesperetin can also be utilized as a useful intermediate for the preparation of sweeter and dye [6]. Because of abundant presence in fruits and a lot of interesting bioactivities of hesperetin, its biodistribution study would provide meaningful biological and pharmacological information for understanding bioavailability and bioactivity as well as for development of new biomolecules including drug candidate, specific biomarker, and food additive. Until now, a few researches about in vivo biodistribution and pharmacokinetics of hesperetin have been published [7–11]. The analysis method in those reports mainly depended on liquid chromatography (LC) along with mass spectroscopy and the results of biological studies demonstrated that hesperetin showed short half-lives in serum and faster elimination from the body.
In recent years, several radioisotopes have been utilized for biodistribution studies of biologically important natural product because nuclear medicine techniques provide a few advantages over conventionally used methods in terms of detection sensitivity and availability. Among them radioiodine is one of the most suitable radioisotope for the labeling of flavonoids because it can be easily introduced to a phenol group on the substrate with a minimal structural change from the original substrate. Several radioiodine-labeled flavonoids have been prepared for the purpose of biodistribution study and (or) specific targeting of disease [12–16]. Importantly, the radioiodine labeled product can be applied to development of PET (124I) or SPECT (123I, 125I) radiotracer, which provides a long-term imaging compared with the substrates labeled with short half-live radioisotopes such as 11C, 18F and 99mTc. Moreover radioiodine labeled product can be developed as a potential therapeutic agent by employing 131I. Considering that flavonoid is normally taken by ingestion of food, biodistribution study of orally administered flavonoid which is labeled with a radioisotope would be a suitable method for its in vivo evaluations. Despite of extensive previous works, quantitative organ distribution and in vivo behavior of orally administered hesperetin has not been fully investigated. In the present study, we synthesized 123I (t 1/2 = 13.22 h) labeled hesperetin with an optimal radiochemical procedure and performed biodistribution studies and SPECT/CT imaging to investigate in vivo behavior of the orally administered hesperetin.
Experimental
Materials and methods
Chloramine T, peracetic acid (32 wt% in diluted acetic acid), sodium metabisulfite, and sodium iodine (non-radioactive) were purchased from Sigma-Aldrich Korea and Phosphoric acid was obtained from DC chemical Co., Ltd. 123I was supplied by New Korea Industrial Co. Ltd. All commercially available reagents were used without purification step. 1H NMR spectra were measured on a JEOL 500 MHz spectrometer with DMSO-d6 as a solvent. Data were reported as follows: chemical shifts are reported as δ in units of parts per million (ppm) relative to an internal standard (tetramethylsilane, 0.0 ppm); multiplicities are reported as follows: s (singlet), d (doublet), dd (doublet of double), or m (multiplet); coupling constants are reported as a J value in Hertz (Hz); the number of protons (n) for a given resonance is indicated nH, and based on the spectral integration values. Mass data were obtained from an Agilent LC–MS/MS system.
Animal
ICR male mice (6-weeks old) were purchased from Orientbio Co., Ltd. (Jeonbuk, Korea republic). All animal protocols in the experiments were approved by the Institutional Animal Ethical Committee and performed in strict compliance with the guidelines prescribed by the committee.
Synthesis of iodohesperetin (1a and 1b)
To a solution of hesperetin (100 mg, 0.33 mmol) in 2 mL of MeOH, chloramine T (93 mg, 0.33 mmol) solution in 1.0 mL of 1 × PBS (pH 7.4) and NaI solution (49.6 mg, 0.33 mmol) in 1.0 mL of 1 × PBS (pH 7.4) was added. The mixture was stirred at room temperature for 5 min and the reaction was quenched by the addition of aqueous sodium metabisulfide solution (0.5 M, 0.3 mL). The crude product was purified with a preparative HPLC (flow rate: 12 mL/min, eluent gradient: 40 % of 0.1 % formic acid containing acetonitrile in 0.1 % formic acid containing H2O for 0–3 min; 40–70 % of 0.1 % formic acid containing acetonitrile in 0.1 % formic acid containing H2O for 3–15 min; 70 % of 0.1 % formic acid containing acetonitrile in 0.1 % formic acid containing H2O for 15–25 min) and two major products (1a and 1b, Scheme 1) were obtained (R t = 10.8 and 12.2 min) as a white solid. The observed chemical yields of the products were 25 % (36 mg) for 1a and 23 % (32 mg) for 1b.
1a: 1H NMR (500 MHz, DMSO-d 6) δ 12.15 (s, 1H), 11.62 (s, 1H), 9.07 (s, 1H), 6.92–6.82 (m, 3H), 6.08 (s, 1H), 5.54 (dd, J = 11.5, 3.1, 1H), 3.74 (s, 3H), 3.20 (dd, J = 17.2, 11.5, 1H), 2.82 (dd, J = 17.2, 11.5, 1H); LC/MS (m/z) calculated for C17H16IO5 + [M + H]+ 427.0, Found: 427.0.
1b: 1H NMR (500 MHz, DMSO-d 6) δ 13.03 (s, 1H), 11.66 (s, 1H), 9.08 (s, 1H), 6.92–6.82 (m, 3H), 6.09 (s, 1H), 5.43 (dd, J = 12.2, J = 3.1, 1H), 3.74 (s, 3H), 3.22 (dd, J = 17.2, 12.2, 1H), 2.73 (dd, J = 17.2, 3.1, 1H); LC/MS (m/z) calculated for C17H16IO5 + [M + H]+ 427.0, Found: 427.6.
Radiosynthesis of 123I-labeled hesperetin ([123I]1a and [123I]1b)
To a solution of hesperetin (1 mg) in 1 mL of dry MeOH, aqueous solution of H3PO4 (0.5 M, 0.5 mL) and peracetic acid (0.4 M, 1.0 mL) were added. 50 mCi of [123I]NaI solution (2.0 mL) was added to the reaction mixture. The reaction was carried out for 5 min at room temperature and was quenched by the addition of aqueous sodium metabisulfide solution (0.5 M, 0.5 mL). The crude product was purified with a preparative HPLC (flow rate: 12 mL/min, eluent gradient: 40 % of 0.1 % formic acid containing acetonitrile in 0.1 % formic acid containing H2O for 0–3 min; 40–70 % of 0.1 % formic acid containing acetonitrile in 0.1 % formic acid containing H2O for 3–15 min; 70 % of 0.1 % formic acid containing acetonitrile in 0.1 % formic acid containing H2O for 15–25 min) and two 123I-labeled products ([123I]1a and [123I]1b, Scheme 1) were obtained. The observed radiochemical yields (decay uncorrected) of the products were 31.0 % (15.5 mCi) for [123I]1a and 38.9 % (19.5 mCi) for [123I]1b.
Formulation of [123I]1a and [123I]1b
After purification of the crude product using a preparative HPLC, each fractions containing the desire products ([123I]1a and [123I]1b) were diluted with 30 mL of deionized water. The diluted solution was loaded with a SepPak C18 cartridge (Waters) which was preconditioned with 5 mL of ethanol and 10 mL of deionized water. The products trapped in the SepPak C18 cartridge were eluted from with 1.5 mL of absolute ethanol and then the ethanol solutions were diluted with 13.5 mL of saline for in vivo experiment.
In vitro stability test of 123I-labeled hesperetin
Each aliquot of the purified products ([123I]1a and [123I]1b, 0.1 mL, 50 μCi) were kept in acidic solution (1.0 mL, pH 1.5) and normal mouse serum (1.0 mL) at room temperature for 24 h. The stability results of each solution were determined by the integration of analytical HPLC results at different time points (0, 1, 2, 4, and 24 h).
Biodistribution study
The 123I-labeled products ([123I]1a and [123I]1b) which were formulated with 10 % ethanol in saline were orally administrated (1 μCi per mouse) to ICR male mice (n = 25 for each product). At each time point (1, 2, 4, 12, and 24 h), five mice were sacrificed under anesthesia and the organs (heart, lungs, liver, spleen, kidneys, stomach, small intestine and large intestine) and the blood were then harvested. The collected organs and blood were weight and then the radioactivity was measured by a use of γ-counter. The raw data were corrected by considering the half-life of 123I. The distribution data were presented as the percentage of injected dose per whole tissue.
SPECT/CT study
SPECT/CT imaging study was performed with oral administration of [123I]1a to animals. ICR mice (20–25 g) were anesthetized with 2 % isoflurane. The 123I-labeled hesperetin (200 μCi in 200 μL) was orally administrated to the mice via a syringe. SPECT/CT images were obtained from a small-animal SPECT/CT system.
Results and discussion
Preparation of [123I]1a and [123I]1b
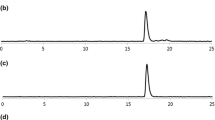
Synthesis of iodohesperetin (1a and 1b) for identification of the 123I-labeled hesperetin was carried out under the well-known iodination protocol using chloramine T and sodium iodide (condition A in Scheme 1). After the reaction was quenched by aqueous sodium metabisulfite, two major products were purified by using a preparative HPLC. 1H NMR and COSY analysis revealed that the first product, which was eluted at earlier retention time from HPLC, was 8-iodohesperetin 1a and the other product was found to be 6-iodohesperetin 1b. LC/MS results of both compounds were well matched with the calculated molecular weight. Radiolabeling of hesperetin (condition B in Scheme 1) with 123I was carried out under acidic condition because chloramine T and higher pH of the condition A resulted in undesired byproducts during the radiolabeling reaction. Alternatively the condition B, which required less than 5 min at room temperature, provided a quite straightforward result and showed high conversion yield (>95 %, based on analytical HPLC integration). As shown in Fig. 1, radiochromatography of the crude product from the condition B showed two major peaks. Two radiolabeled isomers were separable completely by a use of preparative HPLC, and then separation of the crude product gave the purified products ([123I]1a and [123I]1b) which showed more than 99 % of radiochemical purities (n = 5). The isolated radiochemical yields of [123I]1a and [123I]1b were 31 ± 4 % and 39 ± 5 % (n = 3) respectively. The observed peaks detected by UV of 1a and 1b and radioactivity of [123I]1a and [123I]1b in the HPLC chromatogram were overlapped respectively and thus structures of the 123I-labeled products were confirmed by comparison with iodohesperetin 1a and 1b. Different amount of hesperetin (0.5–2.0 mg) also have been tried in the labeling reaction, however the obtained radiochemical yield was not varied. The obtained results from the labeling experiment showed noticeable advances compared with other similar studies. In the previous report about radiosynthesis of hesperetin using 131I, only single product was isolated and it was applied to in vivo experiment [13]. In some cases, the radioiodine labeled product from some polyphenol natural product was not well characterized [16]. In the present study, however, two isomers of radiolabeled hesperetin were purified separately and characterized their structures using spectroscopic data of the non-radioactive products (1a, 1b). The experimental protocol and analysis results used in this study would be a standard method for the radiolabeling of similar natural product.
In vitro stability
Orally administered radiotracer is subjected to gastric acid which pH is generally 1.5 and thus, chemical stability test under acidic condition is necessary for in vivo experiment. The stability test of [123I]1a in the acidic solution (pH 1.5) showed a good stability (>87 %) during 24 h (Fig. 2). The radiochemical purity of [123I]1a in mouse serum was also retained more than 85 % during 24 h. Similar results were obtained with [123I]1b in two different environments (acidic pH and mouse serum) therefore both products were determined to be suitable radiotracers for in vivo biodistribution study.
Biodistribution study
To determine the biodistribution of hesperetin, we have investigated both tracers [123I]1a and [123I]1b. After the formulation step of [123I]1a with 10 % ethanol in saline, it was orally administrated to five groups of mice. Table 1 and Fig. 3 showed the biodistribution data of [123I]1a, which were prepared by uptake values (%ID/g) in mice organs. As shown in the results, most of the radioactivity at the first time point was found in the stomach (51.9 %), small and large intestines (35.7 and 6.09 % respectively). The observed radioactivities of the stomach and small intestine were then decreased while that of large intestine showed its maximum uptake at 3 h after administration. That represented the digestion and excretion process of hesperetin via the gastrointestinal tract. The SPECT/CT imaging data of [123I]1a were well-matched with the biodistribution data from γ-counter, representing that most of the radioactivity were clearly detected in the stomach and intestines at the first time point (1 h) and decreased signals in the organs were observed at 4 h after injection (Fig. 3).
It is noteworthy that significant amounts of [123I]1a were accumulated in liver (4.79 %) and kidneys (1.36 %) and some amount of 123I signals were also detected in blood (0.70 %) at 1 h post injection. The uptake value of liver was then decreased and therefore it was found to be 0.73 % at 24 h after administration. Those results suggested that a potion of [123I]1a was initially absorbed in small intestine and then it might be transferred to liver and other organs through blood stream. The absorbed [123I]1a in the liver might be metabolized and excreted via the bile or the kidney, which showed certain amounts of 123I radioactivity in liver and kidneys. Previous studies showed that hesperetin can be transferred from the GI tract and then it is subsequently absorbed from intestinal mucosa. In the intestinal cells, hesperetin is conjugated by uridine 5′-diphospho-glucuronosyltransferase and sulfotransferase into glucuronidated and sulfonated metabolites respectively. Those metabolites from hesperetin have been found in both rat and human plasma [17, 18]. In addition, the biodistribution result of tail vein injected 131I-labeled hesperetin demonstrated that considerable uptake (1.93 %ID/g) was observed in blood within 20 min and then radioactivity in the blood was quickly distributed from blood to organs such as liver (5.47 %ID/g), lung (5.38 %ID/g) and stomach (5.55 %ID/g) [13]. These reports support our biodistribution data obtained from [123I]1a, which showed significant uptake values in some organs such as liver and kidneys. While the biodistribution study of the isomer, [123I]1b, have also been carried out via oral administration to mice (Table 2). Overall uptake values of [123I]1b in the gastrointestinal tract and other organs were lower than those of [123I]1a. Those results might be attributed to faster metabolism and excretion of [123I]1b than its isomer or low chemical stability of [123I]1b. But distribution trend of [123I]1b was similar with its isomer [123I]1a, which showed high ID %/g values in the stomach, small intestine and large intestine at the first time point (1 h). Considerable accumulations of radioactivity were also observed in the liver (1.74 %) and blood (1.42 %) at 1 h post injection and the uptake values were decreased at later time points.
Reported pharmacokinetic data of hesperetin were normally obtained by spectroscopic analysis of plasma, serum and urine samples [7–11]. On the other hand, the present study utilized radiolabeled tracer that provided quantitative organ biodistribution of hesperetin in different time point. Because radiolabeled hesperetin ([123I]1) was found to be quite stable in serum and acidic conditions (Fig. 2), we believe that the biodistribution data from radiolabeled tracer ([123I]1) was reliable and could provide meaningful data about in vivo behavior of orally administered hesperetin. To the best of our knowledge, the current research is the first report describing quantitative biodistribution of orally administered hesperetin.
Conclusions
In the present study, we described an optimized radiolabeling procedure of hesperetin. Under oxidation condition using peracetic acid, two 123I-labeled products were obtained with high radiochemical yields (>70 %, combined yield of both products) and radiochemical purities (>99 %). Both isomers were isolated separately by radio-HPLC and characterized by spectroscopic data of their standard compounds. The results from biodistribution study and SPECT/CT images of the orally administrated radiotracer showed its in vivo fate in the normal mice. Most of 123I-labeled hesperetin was initially retained in the gastrointestinal tract, and a part of 123I-labeled hesperetin was then absorbed into the bloodstream from the small intestine and then distributed to normal organs including liver, kidneys and lung. Those results would provide valuable information about in vivo behavior of orally administered hesperetin. The radiolabeling and analysis method used in this study will be applied to the radiosynthesis of other flavonoids and natural products.
References
Tomás-Barberán FA, Clifford MN (2000) Flavanones, chalcones and dihydrochalcones—nature, occurrence and dietary burden. J Sci Food Agric 80(7):1073–1080
Manthey JA, Grohmann K (1996) Concentrations of hesperidin and other orange peel flavonoids in citrus processing byproducts. J Agric Food Chem 44(3):811–814
Shin KC, Nam HK, Oh DK (2013) Hydrolysis of flavanone glycosides by β-glucosidase from Pyrococcus furiosus and its application to the production of flavanone aglycones from citrus extracts. J Agric Food Chem 61(47):11532–11540
Yang HL, Chen SC, Kumar KJS, Yu KN, Chao PDL, Tsai SY, Hou YC, Hseu YC (2012) Antioxidant and anti-inflammatory potential of hesperetin metabolites obtained from hesperetin-administered rat serum: an ex vivo approach. J Agric Food Chem 60(1):522–532
Iio A, Ohguchi K, Iinuma M, Nozawa Y, Ito M (2012) Hesperetin upregulates ABCA1 expression and promotes cholesterol efflux from THP-1 macrophages. J Nat Prod 75(4):563–566
Ruen-ngam D, Quitain AT, Sasaki M, Goto M (2012) Hydrothermal hydrolysis of hesperidin into more valuable compounds under supercritical carbon dioxide condition. Ind Eng Chem Res 51(42):13545–13551
Kanaza FI, Bounartzi MI, Georgarakis M, Niopas I (2007) Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur J Clin Nutr 61(4):472–477
Yáñez JA, Remsberg CM, Miranda ND, Vega-Villa KR, Andrew PK, Davies NM (2008) Pharmacokinetics of selected chiral flavonoids: hesperetin, naringenin and eriodictyol in rats and their content in fruit juices. Biopharm Drug Dispos 29(2):63–82
Maiti K, Mukherjee K, Murugan V, Saha BP, Mukherjee PK (2009) Exploring the effect of hesperetin-HSPC complex—a novel drug delivery system on the in vivo release, therapeutic efficacy and pharmacokinetics. AAPS PharmSciTech 10(3):943–950
Srirangam R, Hippalgaonkar K, Avula B, Khan IA, Majumdar S (2012) Evaluation of the intravenous and topical routes for ocular delivery of hesperidin and hesperetin. J Ocul Pharmacol Ther 28(6):618–627
Sun H, Dong T, Zhang A, Yang J, Yan G, Sakurai T, Wu X, Han Y, Wang X (2013) Pharmacokinetics of hesperetin and naringenin in the Zhi Zhu Wan, a traditional Chinese medicinal formulae, and its pharmacodynamics study. Phytother Res 27(9):1345–1351
Barolli MG, Pomilio AB (1997) Synthesis of [131-I]-iodinated quercetin. J Label Compd Radiopharm 39(11):927–933
Kim SK, Ham I, Bu Y, Kim H, Cho JH, Choi H (2008) Study on the attributive channel theory of herbal medicine by the pharmacodynamics research of I-131 labelled hesperetin. Korea J Herbol 23(1):117–125
Seyitoglu B, Lambrecht FY, Durkan K (2009) Labeling of apigenin with 131I and bioactivity of 131I-apigenin in male and female rats. J Radioanal Nucl Chem 279(3):867–873
Lambrecht FY, Yilmaz O, Bayrak E, Kocagozoglu G, Durkan K (2010) Could be radiolabeled flavonoid used to evaluate infection? J Radioanal Nucl Chem 283(2):503–506
Diao Y, Zhao W, Li Y, Liao L, Wang O, Liu J, Zhao X, Yu C, Cui Z (2014) Radiolabeling of EGCG with 125I and its biodistribution in mice. J Radioanal Nucl Chem 301(1):167–173
Masumoto H, Ikoma Y, Sugiura M, Yano M, Hasegawa Y (2004) Identification and quantification of the conjugated metabolites derived from orally administered hesperidin in rat plasma. J Agric Food Chem 52(21):6653–6659
Mullen W, Archeveque MA, Edwards CA, Matsumoto H, Crozier A (2008) Bioavailability and metabolism of orange juice flavanones in humans: impact of a full-fat yogurt. J Agric Food Chem 56(23):11157–11164
Acknowledgments
This work was supported by the National Research Foundation of Korea grant funded by the Korea government (Grant Nos. 2012M2B2B1055245 and 2012M2A2A6011335) and Korea Atomic Energy Research Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeon, J., Ma, SY., Choi, D.S. et al. Radiosynthesis of 123I-labeled hesperetin for biodistribution study of orally administered hesperetin. J Radioanal Nucl Chem 306, 437–443 (2015). https://doi.org/10.1007/s10967-015-4093-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4093-6