Abstract
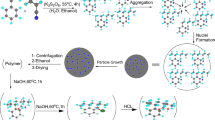
The copolymers of styrene and maleic anhydride resin (PSt/MA) was synthesized by free radical polymerization and characterized by means of FTIR. It is shown that the PSt/MA copolymer has rather strong coordination ability to UO2 2+ ions by chelation with the carboxylate group, and the microstructures of the U(VI)-PSt/MA complexes can be well controlled. The influence factors on UO2 2+ ions were also investigated and described in detail, such as contact time, solid/liquid ratio, pH value, ethanol content, and initial concentration. It was found that the maximum adsorption quantity of UO2 2+ was 831 mg/g. Experiments show that PSt/MA can recover UO2 2+ ions with high adsorption selectively from a simulated industry solution containing Ca2+ and Mg2+ as impurities. The adsorption kinetic data were best described by the pseudo-second-order equation, indicating that the chemical adsorption was the rate-limiting step. And there are very good correlation coefficients of linearized equations for Langmuir model, which indicated that the sorption isotherm of the PSt/MA for UO2 2+ can be fitted to the Langmuir model. After five times of repeated tests for the hydrogel it still remained its excellent adsorption.









Similar content being viewed by others
References
Benedict B, Pigford TH, Levi HW (1981) Nuclear chemical engineering. McGraw-Hill, New York
Eisenbud M, Gesell T (1997) Environmental radioactivity from natural, industrial, and military sources. Academic Press, San Diego
Choppin GR, Morgenstern A (2000) Radionuclide separations in radioactive wastes. Disposal. J Radioanal Nucl Chem 243:45–51
Xie SB, Yang J, Chen C, Zhang XJ, Wang QL, Zhang C (2008) Study on biosorption kinetics and thermodynamics of uranium by Citrobacter freudii. J Environ Radiact 99:126–133
Horn JD, Van Huang H (2006) Uranium(VI) bio-coordination chemistry from biochemical, solution and protein structural data. Coord Chem Rev 250:765–775
Epa US (1996) EPA Integrated Risk Information System (IRIS) electronic database. U.S. Environmental Protection Agency, Washington, DC
Sun X, Huang X, Liao XP, Shi B (2010) Adsorptive recovery of UO2 2+ from aqueous solutions using collagen–tannin resin. J Hazard Mater 179:295–302
Aydin FA, Soylak M (2007) A novel multi-element co precipitation technique for separation and enrichment of metal ions in environmental samples. Talanta 73:134–141
Donia AM, Atia AA, Moussa MM, Sherif AM, Magied MO (2009) Removal of uranium(VI) from aqueous solutions using glycidyl methacrylate chelating resins. Hydrometallurgy 95:183–189
Sodayea H, Nisanb S, Poletikoc C, Prabhakara S, Tewaria PK (2009) Extraction of uranium from the concentrated brine rejected by integrated nuclear desalination plants. Desalination 235:9–32
Kuhu AT (1972) Electrochemistry of cleaner environments. Plenum Press, New York
Lapka JL, Paulenova A, Alyapyshev MY, Babain VA, Herbst RS, Law JD (2009) Extraction of uranium(VI) with diamides of dipicolinic acid from nitric acid solutions. Radiochim Acta 97:291–296
Aydin FA, Soylak M (2007) Solid phase extraction and preconcentration of uranium (VI) and thorium(IV) on Duolite XAD761 prior to their inductively coupled plasma mass spectrometric determination. Talanta 72:187–192
Unuabonah EI, Adebowal KO, Olu-owolabi BI, Yang LZ, Kong LX (2008) Adsorption of Pb (II) and Cd (II) from aqueous solutions onto sodium tetraborate-modified Kaolinite clay: equilibrium and thermodynamic studies. Hydrometallurgy 93:1–9
Wan Ngah WS, Endud CS, Mayanar R (2002) Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React Funct Polym 50:181–190
Wang GH, Liu JS, Wang XG, Xie ZY, Deng NS (2009) Adsorption of uranium (VI) from aqueous solution onto cross-linked chitosan. J Hazard Mater 168:1053–1058
Özeroğlu C, Keçeli G (2006) Removal of strontium ions by a crosslinked copolymer containing methacrylic acid functional groups. J Radioanal Nucl Chem 268:211–219
Özeroğlu C, Doğan E, Keçeli G (2011) Investigation of Cs(I) adsorption on densely crosslinked poly(sodium methacrylate) from aqueous solutions. J Radioanal Nucl Chem 289:577–586
Atia AA, Donia AM, El-Enein SA, Yousif AM (2007) Effect of chain length of aliphatic amines immobilized on a magnetic glycidyl methacrylate resin towards the uptake behavior of Hg(II) from aqueous solutions. Sep Sci Technol 42:403–420
Prabhakaran D, Subramanian MS (2003) A column system for the selective extraction of U(VI) and Th(IV) using a new chelating sorbent. Talanta 61:423–430
Maheswari MA, Subramanian MS (2005) AXAD-16-3,4-dihydroxy benzoyl methyl phosphonic acid: a selective preconcentrator for U and Th from acidic waste streams and environmental samples. React Funct Polym 62:105–114
Qi XH, Jia XQ, Yang Y, Niu LE, Hou LP (2010) Recovery of nickel from mixed solution containing light metals by PSt/MA resin. Trans Nonferrous Met Soc China 20:s102–s106
Huang FQ, Zheng YA, Yang Y (2007) Study on macromolecular metal complexes: synthesis, characterization, and fluorescence properties of stoichiometric complexes for rare earth coordinated with poly(acrylic acid). J Appl Polym Sci 103:351–357
Duan GJ, Yang Y, Cui YM (2006) Study on macromolecular rare earth complexes (IV)—Synthesis, characterization and fluorescent properties of rare earth complexes with polymethyl acrylic acid. Synth React Inorg Met-Org Chem 36:459–463
Wei M, Liao JL, Liu N, Zhang D, Kang HJ, Yang YY, Yong Y, Jin JN (2007) Interaction between uranium and humic acid (I): adsorption behaviors of U(VI) in soil humic acids. Nucl Sci Tech 18:287–293
Qi XH, Jia XQ, Yang Y, Niu LE (2009) Formation and recovery of Co2+, Ni2+, Cu2+ macromolecular complexes with, polystyrene and acrylic acid. Hydrometallurgy 96:269–274
Kakihana M, Nagumo T (1987) Coordination structures for uranyl carboxylate complexes in aqueous solution studied by IR and carbon-13 NMR spectra. J Phys Chem 91:6128–6136
Deacon GB, Phillips R (1980) Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. J Coord Chem Rev 33:227–250
Groenewold GS, de Jong WA, Oomens J, Van Stipdonk MJ (2010) Variable denticity in carboxylate binding to the uranyl coordination complexes. J Am Soc Mass Spectrom 21:719–727
Rivas BL, Maturana HA, Ocampa X, Peric IM (1995) Adsorption behavior of Cu2+ and UO2 2+ ions on crosslinked Poly[2,2-bis(acrylamido)acetic acid]. J Appl Polym Sci 58:2201–2205
Saberi R, Nilchi A, Garmarodi SR, Zarghami R (2010) Adsorption characteristic of 137Cs from aqueous solution using PANbased sodium titanosilicate composite. J Radioanal Nucl Chem 284:461–469
Lu S, Guo Z, Zhang C, Zhang S (2011) Sorption of Th(IV) on MX-80 bentonite: effect of pH and modeling. J Radioanal Nucl Chem 287:621–628
Chen CY, Chen SY (2004) Adsorption properties of a chelating resin containing hydroxy group and iminodiacetic acid for copper ions. J Appl Polym Sci 94:2123–2130
Matilda P, Sanghamitra K, Gladis JM, Naidu GRK, Rao TP (2005) Amberlite XAD-4 functionalized with succinic acid for the solid phase extractive preconcentration and separation of uranium(VI). Talanta 65:192–200
Pekel N, Güven O (2003) Separation of uranyl ions with amidoximated poly(acrylonitrile/N-vinylimidazole) complexing sorbents. Colloids Surf A 212:155–161
Chiou MS, Li HY (2002) Equilibrium and kinetic modeling of adsorption of reactive dye on cross-linked chitosan beads. J Hazard Mater B 93:233–248
Yıldız B, Erten HN, Kıs M (2011) The sorption behavior of Cs+ ion on clay minerals and zeolite in radioactive waste management: sorption kinetics and thermodynamics. J Radioanal Nucl Chem 288:475–483
Bagherifam S, Lakzian A, Ahmadi SJ, Rahimi MF, Halajnia A (2010) Uranium removal from aqueous solutions by wood powder and wheat straw. J Radioanal Nucl Chem 283:289–296
Ren XM, Wang SW, Yang ST, Li JX (2010) Influence of contact time, pH, soil humic/fulvic acids, ionic strength and temperature on sorption of U(VI) onto MX-80 bentonite. J Radioanal Nucl Chem 283:253–259
Ansari SA, Mohapatra PK, Manchanda VK (2007) Synthesis of N, N′-dimethyl-N, N′-dibutyl malonamide functionalized polymer and its sorption affinities towards U(VI) and Th(IV) ions. Talanta 73:878–885
Bhatnagar A, Jain AK (2005) A comparative adsorption study with different industrial wastes as adsorbents for the removal of cationic dyes from water. J Colloid Interface Sci 281:49–55
Kutahyal C, Eral M (2004) Selective adsorption of uranium from aqueous solutions using activated carbon prepared from charcoal by chemical activation. Sep Purif Technol 40:109–114
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. J1030932 and No. J51074083), Specialized Research Fund for the Doctoral Program of Higher Education (No. 20110211120038) and Fundamental Research Funds for the Central Universities (lzujbky-2012-64).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guojian, D., Tonghuan, L., Wangsuo, W. et al. Adsorption of UO2 2+ from aqueous solution onto copolymers of styrene and maleic anhydride. J Radioanal Nucl Chem 295, 2193–2201 (2013). https://doi.org/10.1007/s10967-012-2275-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-2275-z