Abstract
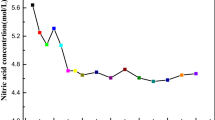
In nuclear technology, tri-n-butyl phosphate (TBP) diluted with a hydrocarbon diluent such as n-dodecane or NPH is the most frequently used solvent in liquid–liquid extraction for fuel reprocessing. This extraction, known as the plutonium uranium refining by extraction, is still considered as the most dominant process for the extraction of uranium and plutonium from irradiated fuels. The solubility of pure TBP in water is about 0.4 g/L at 25 °C. This is enough to create trouble during evaporation of raffinate and product solutions. Solubility data for undiluted TBP and TBP (diluted in inert hydrocarbon diluent) in various concentrations of nitric acid is not adequate in the literature. The solubility data generated in the present study provide complete information on the solubility of TBP in various nitric acid concentrations (0–15.7 M) at room temperature. The effect of heavy metal ion concentration such as uranium and various fission products on the solubility of TBP in nitric acid is also presented. The results obtained from gas chromatographic technique were compared with spectrophotometric technique by converting the organic phosphate into inorganic phosphate. The generated data is of direct relevance to reprocessing applications.






Similar content being viewed by others
References
Ramanujam R (1998) IANCAS Bull 2:14
Schulz WW, Navratil JD (1984) Science and technology of tributyl phosphate, vol 1, Chapter 7. CRS Press Inc., Boca Raton
Nichols GC (1960) Technical Report DP-526, E.I. Du Pont de Nemours & Company, Savannah River Laboratory, Aiken, SC
Lee Hyder M (1996) Nucl Technol 16:327
Robinson RN, Gutowski DM, Yeniscavich W (2003) Report DNFSB/TECH-33, Defense Nuclear Facilities Safety Board, Washington
Maeguchi H, Hayashi K, Matsuoka S, Shimada K (1991) OECD Specialist meeting on safety and risk management in fuel cycle facilities, Tokyo, Japan, October, p 475
Long JT (1978) Engineering for nuclear fuel reprocessing. American Nuclear Society, La Grange, pp 273–326
Benedict M, Pigford TH, Levi HW (1981) Nuclear chemical engineering. McGraw Hill Book Company, New York, p 512
Leroy P (1967) Technical Report ORNL TR 4344, Oak Ridge National Laboratory, Tennessee (Original French Report CEA-R-3207)
Geetha R (1996) Indian chemical engineering congress
Kuno Y, Hina T, Masui J (1993) J Nucl Sci Technol 30:567
Burger LL, Forsman RC (1951) Technical Report HW 20936, Hanford works, General Electric & Co, Richland, Washington
Becker R, Stieglitz L, Bautz H (1981) PWA Report 96-81, Kernforschungszentrum, Karlsruhe, Federal Republic of Germany
Ochsenfeld W, Schon J, Smits D, Tullius E (1976) J Nucl Eng Sci 18:258
Shekhar K, Koganti SB (2000) Nucl Technol 129:279
Shekhar K, Koganti SB (1999) Indian J Chem Technol 6:90
Hyder ML (1994) Report WSRC-TR-94-059, Westing house Savannah River Company, Aiken, South Carolina
Davis W, Mrochek J, Hardy CJ (1966) J Inorg Nucl Chem 28:2001
Davis W, Mrochek J, Judkins RR (1970) J Inorg Nucl Chem 32:1689
Davis WJ, Kibbey AH (1970) Report ORNL-TM-3062, Oak Ridge National Laboratory, Tennessee
Wright A, Hartmann PP (2010) Sep Sci Technol 45:1753
Velavendan P, Ahmed MK, Geetha R (2007) Proceedings of the nuclear and radiochemistry symposium (NUCAR 2007), Vadodara, p 207
Belousov EA (1975) J Phys Chem 49:1695
Ganesh S, Velavendan P, Pandey NK, Ahmed MK, Kamachi Mudali U, Natarajan R (2012) J Radioanal Nucl Chem 293:529
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Velavendan, P., Ganesh, S., Pandey, N.K. et al. Studies on solubility of TBP in aqueous solutions of fuel reprocessing. J Radioanal Nucl Chem 295, 1113–1117 (2013). https://doi.org/10.1007/s10967-012-1945-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-1945-1