Abstract
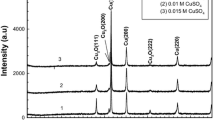
Spherical copper nanoparticles have been prepared by photo- or radiation-induced reduction of aqueous solutions containing 10−3 mol.dm−3 copper sulphate or formate, 1.3 mol.dm−3 propan-2-ol and polyvinyl alcohol as a stabilizer. Increase of initial copper concentration to 10−2 mol.dm−3 resulted in formation of different reaction product—octahedral cuprous oxide nanoparticles. Solutions were irradiated by means of electron beam, 60Co γ rays (dose rate 70 Gy.h−1) or by 400 W medium-pressure mercury lamp and were characterised by UV-Vis spectrophotometry, X-Ray Powder Diffraction, TEM and SEM. Pink to violet colour of colloidal copper solutions corresponded to measured copper surface plasmon band at circa 580 nm and has been found to be very sensitive to oxygen, which causes dissolution of particles. Therefore, the influence of purging by nitrogen gas prior to irradiation was thoroughly examined and has been found to only hinder, not alter irradiation effects. Moreover, the evolution of absorption spectrum of colloidal copper solution in contact with air has been measured, revealing interesting non-monotonous dependence on the air exposure time, probably caused by formation of protective oxide layer. Catalytic activity of prepared cuprous oxide has been measured by catalytic decomposition of hydrogen peroxide and has been found to be higher or comparable to commercial cuprous oxide.









Similar content being viewed by others
References
Belloni J (2006) Catal Today 113:141–156
Rostovshchikova TN, Smirnov VV, Kozhevin VM, Yavsin DA, Zabelin MA, Yassievich IN, Gurevich SA (2005) Appl Catal A 296:70–79
Uhm YR, Park JH, Kim WW, Lee MK, Rhee CK (2007) Mater Sci Eng, A 449–451:817–820
Wang X, Han K, Wan F, Gao Y, Jiang K (2008) Mater Lett 62:3509–3511
Saito M, Yasukawa K, Umeda T, Aoi Y (2008) Opt Mater 30:1201–1204
Zhu HT, Zhang CY, Yin YS (2004) J Cryst Growth 270:722–728
Kanninen P, Johans C, Merta J, Kontturi K (2008) J Colloid Interface Sci 318:88–95
Khatouri J, Mostafavi M, Amblard J, Belloni J (1992) Chem Phys Lett 191:351–356
Zhou R, Wu X, Hao X, Zhou F, Li H, Rao W (2008) Nucl Instrum Methods Phys Res B 266:599–603
Kapoor S, Gopinathan C (1998) Radiat Phys chem 53:165–170
Dey GR (2005) Radiat Phys chem 74:172–184
Joshi SS, Patil SF, Iyer V, Mahumani S (1998) Nanostruct Mater 10:1135–1144
Loginov AV, Gorbunova VV, Boitsova TB (2002) J Nanopart Res 4:193–205
Giuffrida S, Costanzo LL, Ventimiglia G, Bongiorno C (2008) J Nanopart Res 10:1183–1192
Kapoor S, Palit DK, Mukherjee T (2002) Chem Phys Lett 355:383–387
Zhu Y, Qian Y, Zhang M, Chen Z, Xu D, Yang L, Zhou G (1994) Mater Res Bull 29:377–383
He P, Shen X, Gao H (2005) J Colloid Interface Sci 284:510–515
Belloni J, Mostafavi M, Remita H, Marignier JL, Delcourt MO (1998) New J Chem 22:1239–1255
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) J Phys Chem Ref Data 17:513–886
Ershov BG, Janata E, Michaelis M, Henglein A (1991) J Phys Chem 95:8996–8999
Buxton GV, Green JC (1978) J Chem Soc. Faraday Trans 74:697–714
Mira Freiberg, Meyerstein D (1980) J Chem Soc. Faraday Trans 76:1825–1837
Múčka V (1977) Collect Czech Chem Commun 42:2074–2079
ICDD PDF-2 database (1999, version 2.02)
Acknowledgments
This research has been supported by grants MSM 6840770040 and SGS 10/095/OHK4/1T/14. We would also like to thank Mojmír Čamra and Tesla V. T. Mikroel for facilitating electron beam irradiation and Ivo Jakubec from Institute of Inorganic Chemistry, The Academy of Sciences of The Czech Republic for TEM and SEM measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bárta, J., Pospíšil, M. & Čuba, V. Photo- and radiation-induced preparation of nanocrystalline copper and cuprous oxide catalysts. J Radioanal Nucl Chem 286, 611–618 (2010). https://doi.org/10.1007/s10967-010-0748-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0748-5