Abstract
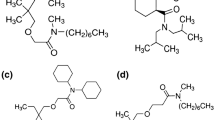
Extraction of microamounts of europium and americium by a nitrobenzene solution of hydrogen dicarbollylcobaltate (H+B−) in the presence of diphenyl-N-butylcarbamoylmethyl phosphine oxide (DPBCMPO, L) has been investigated. The equilibrium data have been explained assuming that the complexes HL+, HL +2 , ML 3+2 , ML 3+3 and ML 3+4 (M3+ = Eu3+, Am3+) are extracted into the organic phase. The values of extraction and stability constants of the species in nitrobenzene saturated with water have been determined. It has been found that the stability constants of the corresponding complexes EuL 3+ n and AmL 3+ n , where n = 2, 3, 4 and L is DPBCMPO, in water saturated nitrobenzene are comparable.
Similar content being viewed by others
References
W. W. Schulz, E. P. Horwitz, Separ. Sci. Technol., 23 (1988) 1191.
C. Cuillerdier, C. Musikas, P. Hoel, L. Nigond, X. Vitart, Separ. Sci. Technol., 26 (1991) 1229.
G. R. Mahajan, D. R. Prabhu, V. K. Manchanda, L. P. Badheka, Waste Managem., 18 (1998) 125.
V. N. Romanovskiy, I. V. Smirnov, V. A. Babain, T. A. Todd, R. S. Herbst, J. D. Law, K. N. Brewer, Solvent Extr. Ion Exch., 19 (2001) 1.
J. D. Law, R. S. Herbst, T. A. Todd, V. N. Romanovskiy, V. A. Babain, V. M. Esimantovskiy, I. V. Smirnov, B. N. Zaitsev, Solvent Extr. Ion Exch., 19 (2001) 23.
E. Makrlík, P. Vaňura, Talanta, 32 (1985) 423.
M. F. Hawthorne, D. C. Young, T. D. Andrews, D. V. Howe, R. L. Pilling, A. D. Pitts, M. Reintjes, L. F. Warren, P. A. Wegner, J. Am. Chem. Soc., 90 (1968) 879.
E. Makrlík, Collect. Czech. Chem. Commun., 57 (1992) 289.
R. Chiarizia, E. P. Horwitz, Solvent Extr. Ion Exch., 10 (1992) 101.
G. Suresh, M. S. Murali, J. N. Mathur, Radiochim. Acta, 91 (2003) 127.
B. J. Mincher, Solvent Extr. Ion Exch., 10 (1992) 615.
P. Vaňura, E. Makrlík, J. Radioanal. Nucl. Chem., 267 (2006) 251.
P. Vaňura, E. Makrlík, J. Radioanal. Nucl. Chem., 267 (2006) 465.
E. Makrlík, P. Vaňura, Collect. Czech. Chem. Commun., 51 (1986) 498.
E. Makrlík, P. Vaňura, P. Selucký, unpublished results.
J. Rais, S. Tachimori, Separ. Sci. Technol., 29 (1994) 1347.
P. Vaňura, E. Makrlík, J. Rais, M. Kyrš, Collect. Czech. Chem. Commun., 47 (1982) 1444.
P. Vaňura, E. Makrlík, Collect. Czech. Chem. Commun., 58 (1993) 1324.
L. G. Sillén, B. Warnqvist, Arkiv Kemi., 31 (1969) 315.
E. Makrlík, P. Vaňura, P. Selucký, J. Radioanal. Nucl. Chem., 275 (2008) 309.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Makrlík, E., Vaňura, P. & Selucký, P. Extraction of microamounts of europium and americium into nitrobenzene by using hydrogen dicarbollylcobaltate in the presence of diphenyl-N-butylcarbamoylmethyl phosphine oxide. J Radioanal Nucl Chem 279, 137–142 (2009). https://doi.org/10.1007/s10967-007-7203-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-007-7203-2