Abstract
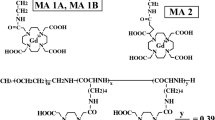
Gd@C82(OH)40 has been developed as a new generation of MRI contrast agent. But recently, it was found that Gd@C82(OH) x with a larger number of OH (x>36) would lead to cage break and hence, release of highly toxic Gd ions. We synthesized the more stable Gd@C82(OH) x with less OH-number, Gd@C82(OH)16, and studied its proton relaxivity and MRI images. The results indicate that Gd@C82(OH)16 also gives high proton relaxivity, even higher than that of (NMG)2-Gd-DTPA. The bio-distribution indicated that Gd@C82(OH)16 tends to be entrapped in the liver and kidney and remained in tissue for about 2 hours. The results suggest that the more stable metallofullerene derivative Gd@C82(OH)16 can be the potential candidate of the new MRI contrast agent.
Similar content being viewed by others
References
J. Y. Huang, X. Y. Liang, Principle of Magnetic Resonance Imaging, Shanxi Science and Technology Press, 1998, p. 150.
F. N. Franano, W. B. Edwards, M. J. Welch, M. W. Brechbiel, O. A. Gansow, J. R. Duncan, Magn. Reson. Imaging., 13 (1995) 201.
P. Wedeking, K. Kumar, M. F. Tweedle, Magn. Reson. Imaging, 10 (1992) 641.
W. P. Cacheris, S. C. Quay, S. M. Rocklage, Magn. Reson. Imaging, 8 (1990) 467.
E. Brücher, in: Contrast Agents I. Magnetic Resonance Imaging, W. Krause (Ed.), Topics in Current Chemistry, Vol. 221, Springer-Verlag, Berlin, 2002, p. 103.
H. W. Kroto, J. R. Heath, S. C. O’Brien, R. F. Curl, R. E. Smalley, Nature, 318 (1985) 162.
R. Taylor, D. R. M. Walton, Nature, 363 (1993) 685.
Shuying Liu, Shuqing Sun, J. Organomet. Chem., 599 (2000) 74.
Jun Tang, Gengmei Xing, Yuliang Zhao, Long Jing, Xingfa Gao, Yue Cheng, Hui Yuan, Feng Zhao, Zhen Chen, Huan Meng, Hui Zhang, Haijie Qian, Run Su, Kurash Ibrahim, Adv. Mater., in press.
Jun Tang, Gengmei Xing, Feng Zhao, Yuliang Zhao, J. Nanosci. Nanotechnol., in press.
D. W. Cagle, S. J. Kennel, S. Mirzadeh, J. M. Alford, L. J. Wilson, Proc. Natl. Acad. Sci. USA, 96 (1999) 5182.
É. Toth, R. D. Bolskar, A. Borel, G. González, L. Helm, A. E. Merbach, B. Sitharaman, L. J. Wilson, J. Am. Chem. Soc., 127 (2005) 799.
B. Sitharaman, R. D. Bolskar, I. Rusakova, L. J. Wilson, Nano Lett., 12 (2004) 2373.
Xing Lu, Jianxun Xu, Zujin Shi, Baoyun Sun, Zhennan Gu, Hongdong Liu, Hongbin Han, Chem. J. Chinese U, 25 (2004) 697.
M. Mikawa, H. Kato, M. Okumura, M. Narazaki, Y. Kanazawa, N. Miwa, H. Shinohara, Bioconjugate Chem., 12 (2001) 510.
R. D. Bolskar, A. F. Benedetto, L. O. Husebo, R. E. Price, E. F. Jackson, S. Wallace, L. J. Wilson, J. M. Alford, J. Am. Chem. Soc., 125 (2003) 5471.
Li Qu, Winbin Cao, Yuliang Zhao, Gengmei Xing, Jun Zhang, Hui Yuan, Jun Tang, Zhifang Chai, Hao Lei, J. Alloy Comp., 408/412 (2006) 400.
Chunying Chen, Gengmei Xing, Jiangxue Wang, Yuliang Zhao, Bai Li, Guang Jia, Tiancheng Wang, Jin Sun, Li Xing, Hui Yuan, Yuxi Gao, Chang Ye, Jun Tang, Li Qu, Huan Meng, Zheng Chen, Zhifang Chai, Nano Lett., 4 (2005) 1050.
Jiangxue Wang, Chunying Chen, Bai Li, Hongwei Yu, Yuliang Zhao, Jin Sun, Yufeng Li, Gengmei Xing, Hui Yuan, Jun Tang, Zhen Chen, Huan Meng, Yuxi Gao, Chang Ye, Zhifang Chai, Chuanfeng Zhu, Baocheng Ma, Xiaohong Fang, Lijun Wan, Biochem. Pharmacol., 71 (2006) 872.
G. M. Xing, J. Zhang, Y. L. Zhao, J. Tang, B. Zhang, X. F. Gao, H. Yuan, L. Qu, W. B. Cao, Z. F. Chai, K. Ibrahim, R. Su, J. Phys. Chem., B108 (2004) 11473.
Yuliang Zhao, Zhenlin Chen, Xinfa Gao, Li Qu, Zhifang Chai, Hui Yuan, Gengmei Xing, Soichiro Yoshimoto, Eishi Tsutsumi, Kingo Itaya, J. Am. Chem. Soc., 126 (2004) 11135.
K. Akiyama, Y. L. Zhao, K. Sueki, K. Tuskada, H. Haba, Y. Nagame, S. Suzuki, T. Otuski, M. Sakaguchi, K. Kikuchi, M. Katada, H. Nakahara, J. Am. Chem. Soc., 123 (2001) 181.
Zhen Chen, Huan Meng, Gengmei Xing, Hui Yuan, Guang Jia, Chunying Chen, Xiaohong Fang, Chang Ye, Zhuanfeng Zhu, Yuliang Zhao, Toxicol Lett., 163 (2006) 109.
Bing Wang, Weiyue Feng, Tian Cheng Wang, Guang Jia, Jun-Wen Shi, Fang Zhang, Yuliang Zhao, Zhifang Chai, Toxicol Lett., 161 (2006) 115.
Haifang Wang, Jing Wang, Xiaoyong Deng, Hongfang Sun, Zujin Shi, Zhennan Gu, Yuanfang Liu, Yuliang Zhao, J. Nanosci. Nanotechnol., 4 (2004) 1019.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, J., Liu, K., Xing, G. et al. Synthesis and in vivo study of metallofullerene based MRI contrast agent. J Radioanal Nucl Chem 272, 605–609 (2007). https://doi.org/10.1007/s10967-007-0632-0
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10967-007-0632-0