Summary
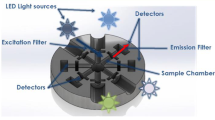
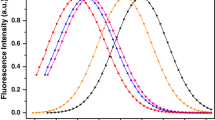
The interaction between the U(VI) and a sodium dodecyl sulfonate (SDS) micelle was studied for intensity and emission lifetime. The measurements of the uranyl ion were done in a 1M H3PO4 medium. The self quenching rate constant (ksq) shows a larger value in micellar than in a SDS monomeric solution. This fact should be interpreted by micelles favoring the localized concentration of UO22+ species. Dynamic and static quenching was observed in the interaction between uranyl ion and the surfactant monomer before the induced critical micelle concentration (icmc) (1 mM) yielding a value of KD = (134±2) . 10M-1 and KS= (16±2) . 102M-1 for the dynamic and static quenching constant, respectively. A quantitative description of the binding was obtained by monitoring the emission lifetime of the uranyl excited state as a function of the surfactant concentration (titration curve), assuming that the observed lifetime is related to a weighted fraction of rate constants for the bound and unbound species.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Repossi, P., Massad, W. & Argüello, G. Interaction of UO22+ with sodium dodecyl sulfonate micelles based on fluorescence data. J Radioanal Nucl Chem 265, 85–90 (2005). https://doi.org/10.1007/s10967-005-0791-9
Issue Date:
DOI: https://doi.org/10.1007/s10967-005-0791-9