Abstract
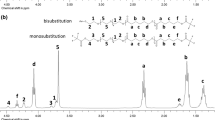
The sequential ring-opening polymerizations (ROP) of ε-caprolactone (ε-CL) and L-lactide (LLA) with benzo-12-crown-4-imidazole carbene (B-12-C-4imY) as the catalyst have been performed. Using either benzyl alcohol or ethylene glycol as an initiator, the corresponding poly(ε-caprolactone)-poly(L-lactide) (PCL-b-PLLA) diblock or poly(L-lactide)-poly(ε-caprolactone)-poly(L-lactide) (PLLA-PCL-PLLA) triblock copolymers were easily prepared. The results indicated that B-12-C-4imY was quite effective for the copolymerization. The diblock copolymerization of ε-CL with LLA could only be achieved when ε-CL was first polymerized followed by LLA. Feeding the two monomers simultaneously, however, only resulted in the formation of LLA homopolymers. Thermogravimetric analysis (TGA) measurements demonstrated that block copolymers exhibited the decomposition temperature lower than the PCL homopolymer. The copolymers were characterized by 1H NMR and 13C NMR, FT-IR, GPC, and DSC analyses. 20 × 10 mm2 rectangular specimens made of the triblock copolymer were allowed to degrade in a pH = 7.4 phosphate buffer at 37 °C. Degradation was monitored by various analytical techniques such as GPC, IR, and ESEM.











Similar content being viewed by others
References
Wang F, Bronich TK, Kabanov AV, Rauh RD, Roovers J (2005) Synthesis and evaluation of a star amphiphilic block copolymer from poly(ε-caprolactone) and poly(ethylene glycol) as a potential drug delivery carrier. Bioconjug Chem 16:397–405
Ho MH, Hou LT, Tu CY, Hsieh HJ, Lai JY, Chen WJ, Wang DM (2006) Promotion of cell affinity of porous PLLA scaffolds by immobilization of RGD peptides via plasma treatment. Macromol Biosci 6:90–98
Meng FL, Zheng SX, Zhang WA, Li HQ, Liang Q (2006) Nanostructured thermosetting blends of epoxy resin and amphiphilic poly(ε-caprolactone)-block-polybutadiene-block-poly(ε-caprolactone) triblock copolymer. Macromolecules 39:711–719
Wang CH, Hsiue GH (2005) Polymer-DNA hybrid nanoparticles based on folate-polyethylenimine-block-poly(L-lactide). Bioconjug Chem 16:391–396
Albertsson AC, Varma IK (2003) Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 4:1466–1486
Arbaoui A, Redshaw C (2010) Metal catalysts for ε-caprolactone polymerization. Polym Chem 1:801–826
Dijkstra PJ, Du HZ, Feijen J (2011) Single site catalysts for stereoselective ring-opening polymerization of lactides. Polym Chem 2:520–527
Pitt CG, Marks TA, Schindler A (1980) Biodegradable drug delivery systems based on aliphatic polyesters: application to contraceptives and narcotic antagonists. In: Baker R (ed) Controlled release of bioactive materials. Academic Press, New York
Riess C, Hurtrez C, Bahadur P (1985) Block copolymers. Encyclopedia of polymer science and engineering 2nd edn. Wiley, New York
Wilson JA, Hopkins SA, Wright PM, Dove AP (2015) Synthesis of ω-pentadecalactone copolymers with independently tunable thermal and degradation behavior. Macromolecules 48:950–958
Dechy-Cabaret O, Martin-Vaca B, Bourissou D (2004) Controlled ring-opening polymerization of lactide and glycolide. Chem Rev 104:6147–6176
Bouyahyi M, Duchateau R (2014) Metal-based catalysts for controlled ring-opening polymerization of macrolactones: high molecular weight and well-defined copolymer architectures. Macromolecules 47:517–524
Pérez Y, del Hierro I, Zazo L, Fernández-Galán R, Fajardo M (2015) The catalytic performance of metal complexes immobilized on SBA-15 in the ring opening polymerization of ε-caprolactone with different metals (Ti, Al, Zn and mg) and immobilization procedures. Dalton Trans 44:4088–4101
Gilmour DJ, Webster RL, Perry MR, Schafer LL (2015) Titanium pyridonates for the homo- and copolymerization of rac-lactide and ε-caprolactone. Dalton Trans 44:12411–12419
Platel RH, Hodgson LM, Williams CK (2008) Biocompatible initiators for lactide polymerization. Polym Rev 48:11–63
Penczek S, Cypryk M, Duda A, Kubisa P, Slomkowski S (2007) Living ring-opening polymerizations of heterocyclic monomers. Prog Polym Sci 32:247–282
Gupta AP, Kumar V (2007) New emerging trends in synthetic biodegradable polymers-polylactide: a critique. Eur Polym J 43:4053–4074
Kamber NE, Jeong W, Waymouth RM, Pratt RC, Lohmeijer BGG, Hedrick JL (2007) Organocatalytic ring-opening polymerization. Chem Rev 107:5813–5840
Ottou WN, Sardon H, Mecerreyes D, Vignolle J, Taton D (2016) Update and challenges in organo-mediated polymerization reactions. Prog Polym Sci 56:64–115
Guillerm B, Lemaur V, Ernould B, Cornil J, Lazzaroni R, Gohy J-F, Dubois P, Coulembier O (2014) A one-pot two-step efficient metal-free process for the generation of PEO-b-PCL-b-PLA amphiphilic triblock copolymers. RSC Adv 4:10028–10038
Makiguchi K, Kikuchi S, Yanai K, Ogasawara Y, Sato S, Satoh T, Kakuchi T (2014) Diphenyl phosphate/4-dimethylaminopyridine as an efficient binary organocatalyst system for controlled/living ring-opening polymerization of L-lacitde leading to diblock and end-functionalized poly(L-lactide)s. J Polym Sci, Part A: Polym Chem 52:1047–1054
Wang X, Liu JQ, Xu SQ, Xu JX, Pan XF, Liu JJ, Cui SD, Li ZJ, Guo K (2016) Tranceless switch organocatalysis enables multiblock ring-opening copolymerizations of lactones, carbonates, and lactides: by a one plus one approach in one pot. Polym Chem 7:6297–6308
Dove AP, Pratt RC, Lohmeijer BGG, Culkin DA, Hagberg EC, Nyce GW, Waymouth RM, Hedrick JL (2006) N-heterocyclic carbenes: effective organic catalysts for living polymerization. Polymer 47:4018–4025
Xiao XD, Bai YL, Liu JQ, Wang JW (2016) Synthesis of novel pillar[5]arene-based N-heterocyclic carbene ligands for Pd-catalysed heck reactions. Tetrahedron Lett 57:3385–3388
Coulembier O, Mespouille L, Hedrick JL, Waymouth RM, Dubois P (2006) Metal-free catalyzed ring-opening polymerization of β-lactones: synthesis of amphiphilic triblock copolymers based on poly(dimethylmalic acid). Macromolecules 39:4001–4008
Raynaud J, Absalon C, Gnanou Y, Taton D (2009) N-heterocyclic carbene-induced zwitterionic ring-opening polymerization of ethylene oxide and direct synthesis of α, ω-difunctionalized poly(ethylene oxide)s and poly(ethylene oxide)-b-poly(ε-caprolactone) block copolymers. J Am Soc 131:3201–3209
Nyce GW, Glauser T, Connor EF, Mock A, Waymouth RM, Hedrick JL (2003) In situ generation of carbenes: a general and versatile platform for organocatalytic living polymerization. J Am Chem Soc 125:3046–3056
Zhang LF, Li N, Wang Y, Guo JZ, Li JF (2014) Ring-opening block copolymerization of ε-caprolactone with L-lactide catalyzed by N-heterocyclic carbenes: synthesis, characteristics, mechanism. Macromol Res 22:600–605
Kamber NE, Jeong W, Gonzalez S, Hedrick JL, Waymouth RM (2009) N-heterocyclic carbene for the organocatalytic ring-opening polymerization of ε-caprolactone. Macromolecules 42:1634–1639
Bai JH, Wu N, Wang Y, Li QR, Wang XQ, Zhang LF (2016) Triblock and pentablock copolymerizations of ε-caprolactone with L-lactide catalyzed by N-heterocyclic carbene. RSC Adv 6:108045–108050
Coulembier O, Lohmeijer BGG, Dove AP, Pratt RC, Mespouille L, Culkin DA, Benight SJ, Dubois P, Waymouth RM, Hedrick JL (2006) Alcohol adducts of N-heterocyclic carbenes: latent catalysts for the thermally-controlled living polymerization of cyclic esters. Macromolecules 39:5617–5628
Acknowledgements
This work was supported by Basic Research Project of Shanxi Province of China (No.2015011029), Undergraduate Innovative Experiment Program of Shanxi Normal University (No.SD2014CXXM-36) and Shanxi Province Education Innovation Project for Postgraduate (No.2015BY38).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Wu, N., Bai, J. et al. Diblock and triblock copolymers catalyzed by benzo-12-crown-4 bridged N-heterocyclic carbene: synthesis, characterization and degradation behavior. J Polym Res 25, 254 (2018). https://doi.org/10.1007/s10965-018-1639-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-018-1639-7