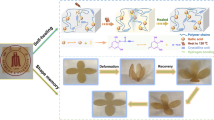
Abstract
A novel small molecule 1,3-bis(eugenyl) glycerol diether is synthesized from renewable eugenol and epichlorohydrin in 60% total yield, and its structure is confirmed by 1H–NMR spectrum. Then, this small molecule is utilized to prepare oligomer, linear polymer and the corresponding crosslinked polymer (denoted as P 2 ) by using thiol-ene and thiol-oxidation reactions. The polymer P 2 can form brown film on a glass substrate and can be easily put off from the substrate. Mechanical properties of P 2 show that tensile strength value is about 6 MPa, with elongation at break of around 300%. Glass transition temperature (Tg) of P 2 is −2.76 °C, meaning that P 2 is at rubber state. There are hydroxyl groups in the prepared linear polymer, which further reacts with 1,6-hexanediisocyanate (HDI) to form polyurethane P 4 with crosslinked structures. Compared with P 2 , the polyurethane P 4 forms yellow film on a glass substrate. But the film of P 4 is not so flexible as that of P 2 , presumably because of relatively higher Tg (5.85 °C) of P 4 than P 2 . Due to the existence of dynamic disulfide bonds as well as hydrogen bonds in both P 2 and P 4 , these thermoset resins show repeatable self-healing behavior stimulated by UV irradiation. Furthermore, the polyurethane P 4 exhibits ultrahigh temperature resistance performance, with Td5 = 375 °C and Td10 = 1000 °C according to TGA curve. This work is expected to expand research and potential applications of the renewable resource eugenol in preparation of smart materials.

















Similar content being viewed by others
References
Gust D, Moore TA, Moore AL (2001). Acc Chem Res 34:40–48
Ashford DL, Gish MK, Vannucci AK, Brennaman MK, Templeton JL, Papanikolas JM, Meyer TJ (2015). Chem Rev 115:13006–13049
Iwata T (2015). Angew Chem Int Ed 54:3210–3215
Chen GQ, Patel MK (2012). Chem Rev 112:2082–2099
Wilbon PA, Chu FX, Tang CB (2013). Macromol Rapid Commun 34:8–37
Qin JL, Liu HZ, Zhang P, Wolcott M, Zhang JW (2014). Polym Int 63:760–765
Cheng CJ, Bai XX, Liu SJ, Huang QH, Tu YM, Wu HM, Wang XJ (2013). J Polym Res 20:197
Han YM, Yuan L, Li GY, Huang LH, Qin TF, Chu FX, Tang CB (2016). Polymer 83:92–100
Kamatou GP, Vermaak I, Viljoen AM (2012). Molecules 17:6953–6981
Rojo L, Vazquez B, Parra J, Bravo AL, Deb S, Roman JS (2006). Biomacromolecules 7:2751–2761
Harvey BG, Sahagun CM, Guenthner AJ, Groshens TJ, Cambrea LR, Reams JT, Mabry JM (2014). ChemSusChem 7:1964–1969
Yoshimura T, Shimasaki T, Teramoto N, Shibata M (2015). Eur Polym J 67:397–408
Harvey BG, Guenthner AJ, Yandek GR, Cambrea LR, Meylemans HA, Baldwin LC, Reams JT (2014). Polymer 55:5073–5079
Dai JY, Jiang YH, Liu XQ, Wang JG, Zhu J (2016). RSC Adv 6:17857–17866
Deng JP, Yang BW, Chen C, Liang JY (2015). ACS Sustain Chem Eng 3:599–605
Hu KL, Zhao DP, Wu GL, Ma JB (2015). Polym Chem 6:7138–7148
Diesendruck CE, Sottos NR, Moore JS, White SR (2015). Angew Chem Int Ed 54:10428–10447
Taylor DL, Panhuis M (2016). Adv Mater 28:9060–9093
Apostolides DE, Patrickios CS, Leontidis E, Kushnir M, Wesdemiotis C (2014). Polym Int 63:1558–1565
Zhang PF, Li GQ (2016). Prog Polym Sci 57:32–63
Sato K, Nakajima T, Hisamatsu T, Nonoyama T, Kurokawa T, Gong JP (2015). Adv Mater 27:6990–6998
Cheng CJ, Zhang X, Chen XH, Li J, Huang QH, Hu ZH, Tu YM (2016). J Polym Res 23:110
Imbernon L, Norvez S (2016). Eur Polym J 82:347–376
White SR, Sottos NR, Geubelle PH, Moore JS, Kessler MR, Sriram SR, Brown EN, Viswanathan S (2001). Nature 409:794–797
Lu YX, Guan ZB (2012). J Am Chem Soc 134:14226–14231
Fickert J, Makowski M, Kappl M, Landfester K, Crespy D (2012). Macromolecules 45:6324–6332
White SR, Moore JS, Sottos NR, Krull BP, WAS C, RCR G (2014). Science 344:620–623
Roy N, Buhler E, Lehn JM (2013). Chem Eur J 19:8814–8820
Kiskan B, Yagci Y (2014). J Polym Sci, Part A: Polym Chem 52:2911–2918
Rao YL, Chortos A, Pfattner R, Lissel F, Chiu YC, Feig V, Xu J, Kurosawa T, Gu XD, Wang C, He MQ, Chung JW, Bao ZN (2016). J Am Chem Soc 138:6020–6027
Chen H, Ma X, Wu SF, Tian H (2014). Angew Chem Int Ed 53:14149–14152
Bai N, Saito K, Simon GP (2013). Polym Chem 4:724–730
Amamoto Y, Kamada J, Otsuka H, Takahara A, Matyjaszewski K (2011). Angew Chem Int Ed 50:1660–1663
Lafont U, van Zeijl H, van der Zwaag S (2012). ACS Appl Mater Interfaces 4:6280–6288
Yang WJ, Tao X, Zhao TT, Weng LX, Kang ET, Wang LH (2015). Polym Chem 6:7027–7035
Michal BT, Jaye CA, Spencer EJ, Rowan SJ (2013). ACS Macro Lett 2:694–699
Amamoto Y, Otsuka H, Takahara A, Matyjaszewski K (2012). Adv Mater 24:3975–3980
Xu WM, Rong MZ, Zhang MQ (2016). J Mater Chem A 4:10683–10690
Morfopoulou CI, Andreopoulou AK, Daletou MK, Neophytides SG, Kallitsis JK (2013). J Mater Chem A 1:1613–1622
Xu CX, Cao YC, Kumar R, Wu X, Wang X, Scott K (2011). J Mater Chem 21:11359–11364
Gao W, Li Z, Zhang D (2002). Oxid Met 57:99–114
Hoyle CE, Bowman CN (2010). Angew Chem Int Ed 49:1540–1573
Yu L, Wang LH, Hu ZT, You YZ, Wu DC, Hong CY (2015). Polym Chem 6:1527–1532
Hong M, Liu SR, Li BX, Li YS (2012). J Polym Sci, Part A: Polym Chem 50:2499–2506
Cheng ZY, Zhang JF, Ballou DP, Williams CH (2011). Chem Rev 111:5768–5783
Acknowledgements
The work was financially supported by Natural Science Foundations of China (NO. 21564004 and 21264008) and Research Fund for Educational Commission of Jiangxi Province of China (No. GJJ150823).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, C., Li, J., Yang, F. et al. Renewable eugenol-based functional polymers with self-healing and high temperature resistance properties. J Polym Res 25, 57 (2018). https://doi.org/10.1007/s10965-018-1460-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-018-1460-3