Abstract
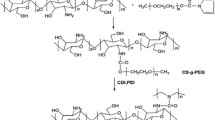
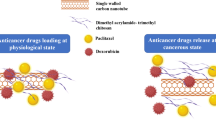
The objective of the research study was to develop and characterize a biodegradable, thermo and pH dual responsive Oxaliplatin-loaded chitosan-graft-poly-N-isopropylacrylamide (CS-g-PNIPAAm) co-polymeric nanoparticles as a tumor-targeting drug delivery system. CS-g-PNIPAAm co-polymers were synthesized, characterized and optimized its thermo and pH responsive properties for tumor microenvironment conditions. Optimized co-polymer could be efficiently loaded with Oxaliplatin in nanoparticle form, evaluated for their morphology (TEM), particle size, zeta potential, loading efficiency and drug content. In vitro drug release study at tumor microenvironment and physiological pH and temperature conditions. The in vitro drug release was optimal at above lower critical solution temperature (LCST) and tumor microenvironment pH when compared to physiological pH & temperature. MTT assay and fluorescence microscopic study showed that drug release and cell uptake was significantly enhanced in tumor microenvironment. In conclusion, the obtained nanoparticles appeared to be of great promise in tumor targeted drug delivery of oxaliplatin.









Similar content being viewed by others
References
Chih-Hao H, Cheng-Fan W, Trong-Ming D, Wen-Yen C (2013) Preparation of pH- and thermosensitive chitosan-PNIPAAm core–shell nanoparticles and evaluation as drug carriers. Cellulose 20:1791–1805
Priya B, Viness P, Yahya EC, du Toit LC (2009) Stimuli-responsive polymers and their applications in drug delivery. Biomed Mater 4:1–15
Fabienne D, Olivier F, Véronique P (2010) To exploit the tumor micro-environment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release 148:135–146
Yin H, Bae YH (2009) Physicochemical aspects of doxorubicin-loaded pH-sensitive polymeric micelle formulations from a mixture of poly(L-histidine)-b-poly(L-lactide)-b-poly(ethylene glycol). Eur J Pharm Sci 71:223–230
Belgacem M, Gandini A (2008) Monomers, polymers and composites from renewable resources, 1st edn. Elsevier Science, Amsterdam
Ru C, Fenghua M, Chao D, Harm-Anton K, Zhiyuan Z (2013) Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 34:3647–3657
Nívia do NM, Ana Maria da SM, Rosangela de CB (2015) Development of dual-sensitive smart polymers by grafting chitosan with poly (N-isopropylacrylamide): an overview. Polímeros 25(3):237–246
Brun-Graeppi AKAS, Richard C, Bessodes M, Scherman D, Merten OW (2010) Thermoresponsive surfaces for cell culture and enzyme-free cell detachment. Prog Polym Sci 35(11):1311–1324
Li F, Wu H, Zhang H, Li F, Yang TH, Gu CH et al (2008) Novel super pH sensitive nanoparticles responsive to tumor extracellular pH. Carbohydr Polym 73(3):390–400
Li F, Wu H, Zhang H, Gu CH, Yang Q (2009) Antitumor drug paclitaxel loaded pH-sensitive nanoparticles targeting tumor extracellular pH. Carbohydr Polym 77(4):773–778
Cunxian D, Dianrui Z, Feihu W, Dandan Z, Lejiao J, Feifei F et al (2011) Chitosan-g-poly(N-isopropylacrylamide) based nanogels for tumor extracellular targeting. Int J Pharm 409:252–259
Rejinold NS, Sreerekha PR, Chennazhi KP, Nair SV, Jayakumar R (2011) Biocompatible, biodegradable and thermo-sensitive chitosan-g-poly (N-isopropylacrylamide) nanocarriers for curcumin drug delivery. Int J Biol Macromol 49(2):161–172
Zhang T, Li G, Guo L, Chen H (2012) Synthesis of thermo-sensitive CS-g-PNIPAM/CMC complex nanoparticles for controlled release of 5-FU. Int J Biol Macromol 51:1109–1115
Wang Y, Wang J, Xu H, Ge L, Zhu J (2015) Investigation of dual-sensitive nanogels based on chitosan and N-isopropylacrylamide and its intelligent drug delivery of 10-hydroxycamptothecine. Drug Deliv 22(6):803–813
Rejinold NS, Chennazhi KP, Nair SV, Jayakumar R (2013) Thermo-responsive chitosan-graft-poly(N-isopropyl acrylamide) co-polymeric nanoparticles for 5-fluorouracil delivery to breast cancer cells in vitro. J Nanopharmaceutics Drug Delivery 1(1):18–29
Jaiswal MK, Pradhan A, Banerjee R, Bahadur D (2014) Dual pH and temperature stimuli-responsive magnetic nanohydrogels for thermo-chemotherapy. J Nanosci Nanotechnol 14(6):4082–4089(8)
Antoniraj MG, Senthil Kumar C, Kandasamy R (2016) Synthesis and characterization of poly (N-isopropylacrylamide)-g-carboxymethyl chitosan copolymer-based doxorubicin-loaded polymeric nanoparticles for thermoresponsive drug release. Colloid Polym Sci 294:527–235
Luo YL, Zhang XY, Fu JY, Xu F, Chen YS (2017) Novel temperature and pH dual sensitive PNIPAM/CMCS/MWCNTs semi-IPN nanohybrid hydrogels: synthesis, characterization and DOX drug release. Int J Polym Mater 66(8):398–409
Abhimanyu P, Shivani S, Bhaskar R, Anne-Laure P, Shiladitya S (2012) Rationally designed oxaliplatin-nanoparticle for enhanced antitumor efficacy. Nanotechnology 23(7):1–17
Tashiro T, Kawada Y, Sakurai Y, Kidani Y (1989) Antitumor activity of a new platinum complex, oxalato (trans-l-1, 2-diaminocyclohexane) platinum (II): new experimental data. Biomed Pharmacother 43(4):251–260
Qiua L, Yanga L, Zhoub H, Longa M, Jianga W, Wanga D, Zhanga X (2012) Encapsulation of oxaliplatin in nanostructured lipid carriers-preparation, physicochemical characterization and in vitro evalulation. Asian. J Pharm Sci 7(5):352–358
Chuang Y, Zhong-Xue F (2014) Liposomal delivery and polyethylene glycol-liposomal oxaliplatin for the treatment of colorectal cancer (review). Biomed Rep 2:335–339
Jingde C, Hong J, Yin W, Yandong L, Yong G (2015) A novel glycyrrhetinic acid-modified oxaliplatin liposome for liver-targeting and in vitro/vivo evaluation. Drug Des Devel Ther 9:2265–2275
Chuang Y, Hai-Zhong L, Zhong-Xue F (2012) Effects of PEG-liposomal oxaliplatin on apoptosis and expression of cyclin a and cyclin D1in colorectal cancer cells. Oncol Rep 28:1006–1012
Chuang Y, Hai ZL, Zhong XF, Wei DL (2011) Oxaliplatin long-circulating liposomes improved therapeutic index of colorectal carcinoma. BMC Biotechnol 11(21):1–8
Qiu L, Yang L, Zhou H, Wang D (2012) Encapsulation of oxaliplatin in nanostructured lipid carriers preparation, physicochemical characterization and in vitro evalulation. Asian J Pharm 7(5):316–324
Linlin W, Changjun M, Hong W, Xiaohe L, Qinghai M, Yu C, Wanshan M (2013) PEGylated multi-walled carbon nanotubes for encapsulation and sustained release of oxaliplatin. Pharm Res 30(2):412–423
Wang K, Liu L, Zhang T, Zhu Y, Qiu F (2011) Oxaliplatin-incorporated micelles eliminate both cancer stem-like and bulk cell populations in colorectal cancer. Int J Nanomedicine 6:3207–3218
Shashank T, Satish Kumar MN, Gowthamarajan K, Ashwati P, Rama Satyanarayana Raju K, Shashank M (2014) Preparation, physicochemical characterization and in vitro evaluation of oxaliplatin solid lipid nanoparticles for the treatment of colorectal cancer. Indo Am J Pharm Res 4(08):3579–3587
Vivek R, Thangam R, Nipunbabu V, Ponraj T, Kannan S (2014) Oxaliplatin-chitosan nanoparticles induced intrinsic apoptotic signaling pathway: a "smart" drug delivery system to breast cancer cell therapy. Int J Biol Macromol 65:289–297
Jonathan PM, Mark JE, Elijus U, Shyh-Dar L (2013) Thermosensitive liposomes for the delivery of gemcitabine and Oxaliplatin to tumors. Mol Pharm 10(12):4499–4508
Jocic D, Tourrette A, Glampedaki P, Warmoeskerken MMCG (2009) Application of temperature and pH responsive microhydrogels for functional finishing of cotton fabric. Mater Technol 24(1):14–23
SelvakumarK YAV (2009) Formulation and evaluation of carvedilol loaded Eudragite 100 nanoparticles. Int J PharmTech Res 1(2):179–183
Chiu YL, Chen SC, Su CJ, Hsiao CW, Chen YM, Chen HL, Sung HW (2009) pH-triggered injectable hydrogels prepared from aqueous N-palmitoyl chitosan: in vitro characteristics and in vivo biocompatibility. Biomaterials 30:4877–4888
Fan L, Hong W, HuiZ FL, Chun-hu G, Qian Y (2009) Antitumor drug paclitaxel-loaded pH-sensitive nanoparticles targeting tumor extracellular pH. Carbohydr Polym 77:773–778
Wang Y, Liu L, Weng J, Zhang Q (2007) Preparation and characterization of self aggregated nanoparticles of cholesterol-modified O-carboxymethyl chitosan conjugates. Carbohydr Polym 69:597–606
Lai JY, Luo LJ (2017) Chitosan-g-poly(N-isopropylacrylamide) copolymers as delivery carriers for intracameral pilocarpine administration. Eur J Pharm Biopharm 113:140–148
Desai N (2012) Challenges in development of nanoparticle-based therapeutics. AAPS J 14:282–295
Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP et al (1998) Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A 95(8):4607–4612
Haleya B, Frenkel E (2008) Nanoparticles for drug delivery in cancer treatment. Urol Oncol 26(1):57–64
Zhang BL, Guo R, Yang M, Jiang X, Liu B (2007) Thermo and pH dual-responsive nanoparticles for anti-cancer drug delivery. Adv Mater 19:2988–2992
Acknowledgements
Authors are thankful to the KLE University, Belagavi for provinding grant to perform this research work. Authors extend their regards to Dr. Prabhakar Kore Basic Science Research Centre, KLE University Belagavi for providing their amenities to carry out this work. The authors are also thankful to Central Institute of Fisheries Technology, Cochin, India for providing chitosan as gift sample.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patil, A.S., Gadad, A.P., Hiremath, R.D. et al. Biocompatible tumor micro-environment responsive CS-g-PNIPAAm co-polymeric nanoparticles for targeted Oxaliplatin delivery. J Polym Res 25, 77 (2018). https://doi.org/10.1007/s10965-018-1453-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-018-1453-2