Abstract
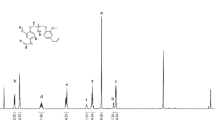
Eugenol, a relatively cheap and abundant renewable resource, is used to design terminal diene compounds. Thiol-ene click reactions between the terminal diene intermediates and 1,6-hexanedithiol afford the corresponding oligomers with molecular mass of about 1.3 kDa. Finally, the oligomers and tricapto compound trimethylolpropane tris(3-mercaptopropionate) undergo thiol-oxidation reactions to form disulfide-crosslinked polymers. The brown polymers indicate self-healable behavior under UV light irradiation, presumably due to reversible reshuffling of weak disulfide bonds and/or reversible addition-dissociation reaction between benzoxazine and thiol groups.













Similar content being viewed by others
References
Iwata T (2015) Biodegradable and bio-based polymers: future prospects of eco-friendly plastics. Angew Chem Int Ed 54:3210–3215
Yao KJ, Tang CB (2013) Controlled polymerization of next-generation renewable monomers and beyond. Macromolecules 46:1689–1712
Wilbon PA, Chu FX, Tang CB (2013) Progress in renewable polymers from natural terpenes, terpenoids, and rosin. Macromol Rapid Commun 34:8–37
Liu R, Zhang XP, Zhu JJ, Liu XY, Wang Z, Yan JL (2015) UV-curable coatings from multiarmed cardanol-based acrylate oligomers. ACS Sustain Chem Eng 3:1313–1320
Cheng CJ, Zhang X, Huang QH, Dou XQ, Li J, Cao XX, Tu YM (2015) Preparation of fully bio-based UV-cured non-isocyanate polyurethanes from ricinoleic acid. J Macromol Sci Part A Pure Appl Chem 52:485–491
Cheng CJ, Bai XX, Liu SJ, Huang QH, Tu YM, Wu HM, Wang XJ (2013) UV cured polymer based on a renewable cardanol derived RAFT agent. J Polym Res 20:197
Voirin C, Caillol S, Sadavarte NV, Tawade BV, Boutevinab B, Wadgaonkar PP (2014) Functionalization of cardanol: towards biobased polymers and additives. Polym Chem 5:3142–3162
Jahangiri M, Bagheri M, Farshi F, Abbasi F (2015) Optimized synthesis of hydroxypropyl cellulose-g-poly(ε-caprolactone) network. J Polym Res 22:196
Shibata M, Tetramoto N, Imada A, Neda M, Sugimoto S (2013) Bio-based thermosetting bismaleimide resins using eugenol, bieugenol and eugenol novolac. React Funct Polym 73:1086–1095
Dumasa L, Bonnauda L, Olivierb M, Poortemanb M, Dubois P (2015) Bio-based high performance thermosets: Stabilization and reinforcement of eugenol-based benzoxazine networks with BMI and CNT. Eur Polym J 67:494–502
Periyasamy T, Asrafali SP, Muthusamy S (2015) New benzoxazines containing polyhedral oligomeric silsesquioxane from eugenol, guaiacol and vanillin. New J Chem 39:1691–1702
Rojo L, Vazquez B, Parra J, Bravo AL, Deb S, Roman JS (2006) From natural products to polymeric derivatives of “eugenol”: a new approach for preparation of dental composites and orthopedic bone cements. Biomacromolecules 7:2751–2761
Qin JL, Liu HZ, Zhang P, Wolcott M, Zhang JW (2014) Use of eugenol and rosin as feedstocks for biobased epoxy resins and study of curing and performance properties. Polym Int 63:760–765
Thirukumaran P, Shakila A, Muthusamy S (2014) Synthesis and characterization of novel bio-based benzoxazines from eugenol. RSC Adv 4:7959–7966
Deng JP, Yang BW, Chen C, Liang JY (2015) Renewable eugenol-based polymeric oil-absorbent microspheres: preparation and oil absorption ability. ACS Sustain Chem Eng 3:599–605
Wei Z, Yang JH, Zhou JX, Xu F, Zrínyi M, Dussault PH, Osadag Y, Chen YM (2014) Self-healing gels based on constitutional dynamic chemistry and their potential applications. Chem Soc Rev 43:8114–8131
Benight SJ, Wang C, Tok JBH, Bao ZN (2013) Stretchable and self-healing polymers and devicesfor electronic skin. Prog Polym Sci 38:1961–1977
Billiet S, Hillewaere XKD, Teixeira RFA, Du Prez FE (2013) Chemistry of crosslinking processes for self-healing polymers. Macromol Rapid Commun 34:290–309
Nguyen LTT, Nguyen HT, Truong TT (2015) Thermally mendable material based on a furyl-telechelic semicrystalline polymer and a maleimide crosslinker. J Polym Res 22:186
Guimard NK, Oehlenschlaeger KK, Zhou JW, Hilf S, Schmidt FG, Barner-Kowollik C (2012) Current trends in the field of self-healing materials. Macromol Chem Phys 213:131–143
Scott TF, Schneider AD, Cook WD, Bowman CN (2005) Photoinduced plasticity in cross-linked polymers. Science 308:1615–1617
Michal BT, Jaye CA, Spencer EJ, Rowan SJ (2013) Inherently photohealable and thermal shape-memory polydisulfide networks. ACS Macro Lett 2:694–699
Amamoto Y, Kamada J, Otsuka H, Takahara A, Matyjaszewski K (2011) Repeatable photoinduced self-healing of covalently cross-linked polymers through reshuffling of trithiocarbonate units. Angew Chem Int Ed 50:1660–1663
Cash JJ, Kubo T, Bapat AP, Sumerlin BS (2015) Room-temperature self-healing polymers based on dynamic-covalent boronic esters. Macromolecules 48:2098–2106
Yu L, Wang LH, Hu ZT, You YZ, Wu DC, Hong CY (2015) Sequential Michael addition thiol–ene and radical-mediated thiol–ene reactions in one-pot produced sequence-ordered polymers. Polym Chem 6:1527–1532
Zaquen N, Wenn B, Ranieri K, Vandenbergh J, Junkers T (2014) Facile design of degradable poly(β-thioester)s with tunable structure and functionality. J Polym Sci Part A Polym Chem 52:178–187
Canadell J, Goossens H, Klumperman B (2011) Self-healing materials based on disulfide links. Macromolecules 44:2536–2541
Cheng CJ, Bai XX, Zhang X, Li HX, Huang QH, Tu YM (2015) Self-healing polymers based on a photo-active reversible addition-fragmentation chain transfer (RAFT) agent. J Polym Res 22:46
Truong VX, Dove AP (2013) Organocatalytic, regioselective nucleophilic “click” addition of thiols to propiolic acid esters for polymer–polymer coupling. Angew Chem Int Ed 52:4132–4136
Hoyle CE, Lowe AB, Bowman CN (2010) Thiol-click chemistry: a multifaceted toolbox for small molecule and polymer synthesis. Chem Soc Rev 39:1355–1387
Lin YM, Lu GP, Cai C, Yi WB (2015) An odorless thia-Michael addition using Bunte salts as thiol surrogates. RSC Adv 5:27107–27111
Xu JT, Boyer C (2015) Visible light photocatalytic thiol–ene reaction: an elegant approach for fast polymer postfunctionalization and step-growth polymerization. Macromolecules 48:520–529
Witt D (2008) Recent developments in disulfide bond formation. Synthesis 2491–2509
Men WW, Lu ZJ (2007) High performance polybenzoxazines. Prog Chem 19:779–786
Hao GP, Li WC, Qian D, Wang GH, Zhang WP, Zhang T, Wang AQ, Schüth F, Bongard HJ, Lu AH (2011) Structurally designed synthesis of mechanically stable poly(benzoxazine-co-resol)-based porous carbon monoliths and their application as high-performance CO2 capture sorbents. J Am Chem Soc 133:11378–11388
Calò E, Maffezzoli A, Mele G, Martina F, Mazzetto SE, Tarzia A, Stifani C (2007) Synthesis of a novel cardanol-based benzoxazine monomer and environmentally sustainable production of polymers and bio-composites. Green Chem 9:754–759
Kawaguchi AW, Sudo A, Endo T (2013) Polymerization–depolymerization system based on reversible addition-dissociation reaction of 1,3-benzoxazine with thiol. ACS Macro Lett 2:1–4
Acknowledgments
The work was financially supported by Natural Science Foundations of China (NO. 21264008, 21564004) and the 8th Foundation of Creativity and Study for College Students in Jiangxi Science Technology Normal University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, C., Zhang, X., Chen, X. et al. Self-healing polymers based on eugenol via combination of thiol-ene and thiol oxidation reactions. J Polym Res 23, 110 (2016). https://doi.org/10.1007/s10965-016-1001-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-016-1001-x