Abstract
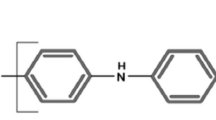
A series of electroactive polyimides with naphthyldiphenylamine units were prepared from the polycondensation reactions of N,N′-bis(4-aminophenyl)-N,N′-di-2-naphthyl-1,4-phenylenediamine with four tetracarboxylic dianhydrides via a conventional two-step technique. Most of the polyimides were readily soluble in many organic solvents and could be solution-cast into tough and amorphous films. These polyimides exhibited glass-transition temperatures of 288–329 °C and did not show significant decomposition before 500 °C. They showed well-defined and reversible redox couples during both p- and n-doping processes, together with multi-electrochromic behaviors.

Anodic and cathodic electrochromism of the cast film of polyimide 4b on an ITO-coated glass substrate











Similar content being viewed by others
References
Wilson D, Stenzenberger HD, Hergenrother PM (eds) (1990) Polyimides. Blackie, Glasgow and London
Sroog CE (1991) Prog Polym Sci 16:561–694
Ghosh MK, Mittal KL (eds) (1996) Polyimides: fundamentals and applications. Marcel Dekker, New York
Liaw DJ, Wang KL, Huang YC, Lee KR, Lai JY, Ha CS (2012) Prog Polym Sci 37:907–974
de Abajo J, de la Campa JG (1999) Adv Polym Sci 140:23–59
Ding M (2007) Prog Polym Sci 32:623–668
Dhara MG, Banerjee S (2010) Prog Polym Sci 35:1022–1077
Harris FW, Hsu SLC (1989) High Perform Polym 1:3–16
Chung IS, Kim SY (2000) Macromolecules 33:3190–3193
Chou CH, Reddy DS, Shu CF (2002) J Polym Sci Part A: Polym Chem 40:3615–3621
Hsiao SH, Lin KH (2005) J Polym Sci Part A: Polym Chem 43:331–341
Kwak SM, Yeon JH, Yoon TH (2006) J Polym Sci Part A: Polym Chem 44:2567–2578
Chern YT, Tsai JY, Wang JJ (2009) J Polym Sci Part A: Polym Chem 47:2443–2452
Chung CW, Lin CH, Cheng PW, Hwang HJ, Dai SA (2009) J Polym Sci Part A: Polym Chem 47:2486–2499
Calle M, Lozano AE, de la Campa JG, de Abajo J (2010) Macromolecules 43:2268–2275
Hsiao SH, Wang HM, Chen WJ, Lee TM, Leu CM (2011) J Polym Sci Part A: Polym Chem 49:3109–3120
Hou YJ, Chen GF, Pei XL, Fang XZ (2012) J Polym Res 19:9955
Hsiao SH, Wang HM, Chang PC, Kung YR, Lee TM (2013) J Polym Sci Part A: Polym Chem 51:2925–2938
Wienk MM, Janssen RAJ (1997) J Am Chem Soc 119:4492–4501
Ito A, Ino H, Tanaka K, Kanemoto K, Kato T (2002) J Org Chem 67:491–498
Fukuzaki E, Nishide H (2006) Org Lett 8:1835–1838
Ito A, Sakamaki D, Ichikawa Y, Tanaka K (2011) Chem Mater 23:841–850
Tang CW, VanSlyke SA (1987) Appl Phys Lett 51:913–915
Shirota Y (2000) J Mater Chem 10:1–25
Shirota Y (2005) J Mater Chem 15:75–93
Shirota Y, Kageyama H (2007) Chem Rev 107:953–1010
Thelakkat M (2002) Macromol Mater Eng 287:442–461
Cheng SH, Hsiao SH, Su TH, Liou GS (2005) Macromolecules 38:307–316
Liou GS, Hsiao SH, Chen HW (2006) J Mater Chem 16:1831–1842
Chang CW, Liou GS, Hsiao SH (2007) J Mater Chem 17:1007–1015
Wang HM, Hsiao SH (2009) Polymer 50:1692–1699
Kung YC, Lee WF, Hsiao SH, Liou GS (2011) J Polym Sci Part A: Polym Chem 49:2210–2221
Hsiao SH, Liou GS, Kung YC, Yen HJ (2008) Macromolecules 41:2800–2808
Kung YC, Liou GS, Hsiao SH (2009) J Polym Sci Part A: Polym Chem 47:1740–1755
Hsiao SH, Liou GS, Kung YC, Hsiung TJ (2010) J Polym Sci Part A: Polym Chem 48:3392–3401
Wang HM, Hsiao SH (2011) J Polym Sci Part A: Polym Chem 49:337–351
Hsiao SH, Wang HM, Chang PC, Kung YR, Lee TM (2013) J Polym Res 20:154
Yen HJ, Liou GS (2012) Polym Chem 3:255–264
Wang YF, Chen TM, Okada K, Uekawa M, Nakaya T, Kitamura M, Inoue H (2000) J Polym Sci Part A: Polym Chem 38:2032–2040
Lambert C, Noll G (1999) J Am Chem Soc 121:8434–8442
Acknowledgments
The financial support of the National Science Council in Taiwan is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsiao, SH., Yeh, SJ., Wang, HM. et al. Synthesis and optoelectronic properties of polyimides with naphthyldiphenylamine chromophores. J Polym Res 21, 407 (2014). https://doi.org/10.1007/s10965-014-0407-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-014-0407-6