Abstract
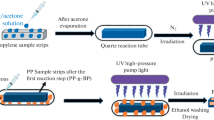
Graft polymerization of N-vinylcaprolactam (VCL) onto the surface of a polylactic acid (PLA) film was performed using a versatile and nondestructive technique. The method used consists of two steps. In the first step, a surface initiator is formed on a substrate under UV irradiation in the presence of a benzophenone (BP) solution, which promotes photoinitiation under UV irradiation at room temperature and under nitrogen. In the second step, the monomer (VCL) is grafted onto the substrate (PLA) by a living polymerization initiated by the surface photoinitiator. The hydrophobic polylactic acid film (PLA) became hydrophilic after grafting; the contact angle of the modified surface decreased drastically from the original value of 77° to <58° within 50 s of irradiation. The surface photografting parameters [polymerization rate (C p), grafting percentage (C g), and grafting efficiency (E g)] were derived using a gravimetric method in which the grafting yield could be controlled by varying the irradiation time or the monomer concentration. The first-derivative UV spectrum recorded between 200 and 360 nm was used as a sensitive tool to follow the grafting process at different times; the signals from VCL at 265 and 310 nm increased in size as the irradiation time increased due to the increase in VCL polymer units during the grafting process. FTIR-ATR analysis exhibited a clearly defined absorption band at 1,620–1,640 cm−1, corresponding to C=O stretching vibrations in the amide groups of unstrained rings in poly(N-vinylcaprolactam) (PNVCL). These analytical techniques confirmed the successful surface graft polymerization of VCL onto the polylactic acid film.










Similar content being viewed by others
References
Gunatillake PA, Adhikari R (2003) Eur Cell Mater 5:1–16
Chromecek RC, Friends GD, Wissman LY, Yourd RA (1984) US Patent No. 4436887
Krish YE, Yanul NA, Popkov YM (2002) Eur Polym J 38(2):403–406
Lozinsky VI, Simenel IA, Kurskaya EA, Kulakova VK, Galaev IYu, Mattiasson B, Grinberg VYa, Grinberg NV, Khokhlo AR (2000) Polymer 41(17):6507–6518
Kumar A, Bansal V, Nandakumar KS, Galaev IY, Roychoudhury PK, Holmdahl R (2006) Biotechnol Bioeng 93:636–646
Kumar A, Caballero AR, Plieva FM, Galaev IYu, Nandakumar KS, Kamihira M, Holmdahl R, Orfao A, Mattiasson B (2005) J Mol Recognit 18:84–93
Kudryavtsev VN, Kabanov VY, Yamul NA, Kedik SA (2003) High Energ Chem 37:382–388
Lunt J (1998) Polym Degrad Stab 59:145–152
Mehta R, Kumar V, Bhunia H, Upadhyay NS (2005) J Macromol Sci Part C 45:325–349
Ohkita T, Lee SH (2006) J Appl Polym Sci 100:3009–3017
Auras R, Harte B, Selke S (2005) In: Proc 63rd Annu Tech Conf Exhibit of the Society of Plastics Engineers, Boston, MA, USA, 1–5 May 2005, pp 3240–3244
Agrawal A, Saran AD, Rath SS, Khana A (2004) Polymer 45:8603–8612
Perego G, Cella GD, Bastioli C (1996) J Appl Polym Sci 59:37–43
Janorkar AV, Metters AT, Hirt DE (2004) Macromolecules 37:9151–9159
Edlund U, Källrot M, Albertsson AC (2005) J Am Chem Soc 127:8865–8871
Gutiérrez-Villarreal MH, Ulloa-Hinojosa M, Gaona-Lozano JG (2008) J Appl Polym Sci 21:163–169
Källrot M, Edlund U, Albertsson AC (2007) Biomacromoleculas 8:2492–2496
Yang WT, Ranby B (1996) Macromolecules 29:3308–3310
Chang-Min X, Yang WT, Jian-Ping D (2005) Macromol Chem Phys 206:1106–1113
Garbassi F, Morra M, Occhiello E (1998) Polymer surfaces: from physics to technology. Wiley, New York
Meeussen F, Nies E, Berghmans H, Verbrugghe S, Goethals EF, Prez D (2000) Polymer 41:8597–8602
Weiser WE, Pardue HL (1983) Clin Chem 29(9):1673–1677
Ma H, Davis RH, Bowman CN (2000) Macromolecules 33:331–335
Kirsh YuE (1998) Poli-N-vinilpirrolidon i drugie poli-N-vinilamidy [Poly(N-vinylpyrrolidone and other poly(N-vinylamide)s]. Nauka, Moscow, p 133
Smith A (1982) Prikladnaya IK-spektroskopiya polimerov. Mir, Moscow, pp 308–331 (translation of: Applied infrared spectroscopy, Wiley, New York)
Acknowledgments
The authors express their gratitude to CONACYT for their financial support of this project (S53075Y).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gutiérrez-Villarreal, M.H., Guzmán-Moreno, J.G. Surface graft polymerization of N-vinylcaprolactam onto polylactic acid film by UV irradiation. J Polym Res 20, 149 (2013). https://doi.org/10.1007/s10965-013-0149-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-013-0149-x