Abstract
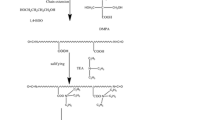
The nano-SiO2 is used to modify the waterborne polyurethane, and the morphology and performance of the waterborne polyurethane (are studied in) prepared by the in-suit polymerization method and the blending method. The properties and structure have been characterized by fourier transform infrared spectra (IR), differential scanning calorimetry (DSC), Thermal gravimetric (TG), transmittance electron microscopy (TEM) and Dynamical Mechanical Analysis(DMA). The experiment results show that, compared with the blending method, the in-suit polymerization has more advantages in that the nano-SiO2 is evenly dispersed in the waterborne polyurethane, obviously in microphase separation and better in resistance to high temperature and water.





Similar content being viewed by others
Abbreviations
- DBTDL:
-
dibutyltin dilaureate
- DSC:
-
Differential Scanning Calorimetry
- DHPDMS:
-
dihydroxy polydimethylsiloxane
- DMPA:
-
dimethylol-propionic acid
- DMA:
-
Dynamical Mechanical Analysis
- EAC:
-
ethyl acetate
- FTIR:
-
Fourier Transform Infrared Spectra
- IPDI:
-
isophorone diisocyanate
- MEK:
-
Methyl ethylketone
- NMP:
-
N-methyl-pyrolidone
- PCL:
-
polycapro- lactone
- PCD:
-
polycarbonate diol
- WPU:
-
polyurethane
- TEA:
-
triethylamine
- TGA:
-
Thermogravimetric Analysis
- TEM:
-
Transmittance Electron Microscopy
- IPDA:
-
isophorone diamine
References
Harjunalanen T, Lahtinen M (2003) The effects of altered reaction conditions on the properties of anionic poly(urethane-urea) dispersions and films cast from the dispersions. Eur Polym J 39:817–824. doi:10.1016/S0014-3057(02)00279-3
Huybrechts J, Bruylants P, Vaes A, De A (2000) Surfactant-free emulsions for waterborne, two-component polyurethane coatings. Prog Org Coat 38:67–77 doi:10.1016/S0300-9440(00)00083-7
Wicks ZW, Wicks DA, Rosthauser JW (2002) Two package waterborne urethane systems. Prog Org Coat 44:161–183. doi:10.1016/S0300-9440(02)00002-4
Hou MH, Liu WQ, Li Y, Chen JH (2005) Waterborne polyurethane doubly modified by montmorillonite and siloxane. Yingyong HuaxueChin 22:1132–1136
Hou MH, Liu WQ, Li Y, Chen JH (2005) Preparation and characterization of waterborne polyurethane/silane montmorillonite nanocomposite. Shiyou HuagongChin 34:677–680
Kuan HC, Ma CC, Chang WP, Yuen SM, Wu HH, Lee TM (2005) Synthesis, thermal, mechanical andrheological properties of multiwall carbon nanotubewaterborne polyurethane nanocomposite. Compos Sci Technol 65:1703–1710. doi:10.1016/j.compscitech.2005.02.017
Kuan HC, Chuang WP, Ma CC (2005) Synthesis and characterization of a clay/waterborne polyurethane nanocomposite. J Mater Sci 40:179–185. doi:10.1007/s10853-005-5704-3
Jeong HM, Jang KH, Cho K (2003) Properties of waterborne polyurethanes based on polycarbonate diol reinforced with organophilic clay, journal of macromolecular science. Physics B 42:1249–1263
Kwon JY, Kim HD (2005) Preparation and properties of acid-treated multiwalled carbon nanotube/waterborne polyurethane nanocomposites. J Appl Polym Sci 96:595–604. doi:10.1002/app.21436
Mamunya YeP, Shtompel VI, Lebedev EV, Pissis P, Kanapitsas A, Boiteux G (2004) Structure and water sorption of polyurethane nanocomposites based on organic and inorganic components. Eur Polym J 40:2323–2331. doi:10.1016/j.eurpolymj.2004.06.007
Kuan HC, Su HY, Ma CC (2005) Synthesis and characterization of polysilicic acid nanoparticles/waterborne polyurethane nanocomposite. J Mater Sci 40:6063–6070. doi:10.1007/s10853-005-1302-7
Yano S, Hick K, Kurita K (1998) Physical properties and structure of organic–inorganic hybrid materials produced by sol–gel process. Mater Sci Eng C 6:75–90. doi:10.1016/S0928-4931(98)00043-5
Jesionowski T (2002) Krysztaf kiewicz, A, preparation of the hydrophilic/hydrophobic silica particles, colloids surf A. physicochem. Eng Aspects 207:49–58. doi:10.1016/S0927-7757(02)00137-1
Cong SF, Yu LR (2003) Polyurethane coating, firsted, chemistry industrial publisher ltd., Perkin. Chapter 11:288
Chen YC, Zhou S, Yang HH, Gu GX, Wu LM (2004) Preparation and characterization of nanocomposite polyurethane. J Colloid Interface Sci 279:370–378. doi:10.1016/j.jcis.2004.06.074
Zhou SX, Wu LM, Sun J, Shen WD (2002) The change of the properties of acrylic-based polyurethane via addition of nano-silica. Prog Org Coat 45:33–42. doi:10.1016/S0300-9440(02)00085-1
Chen TK, Tien YI, Wei KH (2000) Synthesis and characterization of novel segmented polyurethane/clay nanocomposites. Polymer (Guildf) 41:1345–1353. doi:10.1016/S0032-3861(99)00280-3
Acknowledgements
The authors are grateful for the financial support from the Natural Science Foundation of Hubei Province in China (Grants 2006ABA174).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, JJ., Zhu, CF., Deng, HT. et al. Preparation and characterization of the waterborne polyurethane modified with nanosilica. J Polym Res 16, 375–380 (2009). https://doi.org/10.1007/s10965-008-9238-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-008-9238-7