Abstract
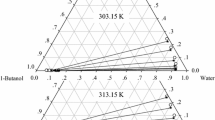
Liquid–liquid equilibrium (LLE) date for the ternary systems of {water + 1-propanol + dibutyl ether (DBE)} and (water + 1-butanol + DBE) were determined at T = (293.15, 303.15, 308.15) K under atmospheric pressure. Distribution coefficients and separation factors of 1-propanol in the mixtures were calculated and are discussed. The influence of temperature on the liquid phase regions was analyzed. In addition, the experimental values were correlated well with the modified and extended UNIQUAC models; the modified UNIQUAC model represents the data better than the extended UNIQUAC model.





Similar content being viewed by others
References
Tynjälä, P., Pakkanen, T.T., Mustamäki, S.: Modification of ZSM-5 zeolite with trimethyl phosphite. 2. Catalytic properties in the conversion of C1–C4 alcohols. J. Phys. Chem. B 102, 5280–5286 (1998)
Demirel, Y.: Estimation of the entropy of vaporization at the normal boiling point for azeotropic mixtures containing water, alcohol or acetic acid. Thermochim. Acta 339, 79–85 (1999)
Cháfer, A., de la Torre, J., Lladosa, E., Montón, J.B.: Liquid–liquid equilibria of 4-methyl-2-pentanone + 1-propanol or 2-propanol + water ternary systems: measurements and correlation at different temperatures. Fluid Phase Equilib. 361, 23–29 (2014)
Çehreli, S., Özmen, D., Dramur, U.: (Liquid + liquid) equilibria of (water + 1-propanol + solvent) at T = 298.2 K. Fluid Phase Equilib. 239, 156–160 (2006)
Ghanadzadeh, H., Ghanadzadeh, A., Bahrpaima, K.: Measurement and prediction of tie-line data for mixtures of (water + 1-propanol + diisopropyl ether): LLE diagrams as a function of temperature. Fluid Phase Equilib. 277, 126–130 (2009)
Merzougui, A., Hasseine, A., Kabouche, A., Korichi, M.: LLE for the extraction of alcohol from aqueous solutions with diethyl ether and dichloromethane at 293.15 K, parameter estimation using a hybrid genetic based approach. Fluid Phase Equilib. 309, 161–167 (2011)
Wypych, G.: Handbook of Solvents. ChemTec Publishing, Toronto (2001)
Wang, C., Guo, J., Cheng, K., Chen, Y.: Ternary (liquid + liquid) equilibria for the extraction of ethanol, or 2-propanol from aqueous solutions with 1,1′-oxybis (butane) at different temperatures. J. Chem. Thermodyn. 94, 119–126 (2016)
Park, S.J., Hwang, I.C., Kwak, H.Y.: Binary liquid–liquid equilibrium (LLE) for dibutyl ether (DBE) + water from (288.15 to 318.15) K and ternary LLE for systems of DBE + C1–C4 alcohols + water at 298.15 K. J. Chem. Eng. Data 53, 2089–2094 (2008)
Arce, A., Rodríguez, H., Rodríguez, O., Soto, A.: (Liquid + liquid) equilibrium of (dibutyl ether + methanol + water) at different temperatures. J. Chem. Thermodyn. 37, 1007–1012 (2005)
Tamura, K., Chen, Y., Tada, K., Yamada, T., Nagata, I.: Representation of multicomponent liquid–liquid equilibria for aqueous and organic solutions using a modified UNIQUAC model. J. Solution Chem. 29, 463–488 (2000)
Nagata, I.: Modification of the extended UNIQUAC model for correlating quaternary liquid–liquid equilibria data. Fluid Phase Equilib. 54, 191–206 (1990)
Barton, A.F.M. (ed): IUPAC Solubility Data Series, Vol. 15. Alcohols with Water. Pergamon Press (1984) https://srdata.nist.gov/solubility/ Accessed March 8, 2018
Stephenson, R., Stuart, J.: Mutual binary solubilities: water–alcohols and water–esters. J. Chem. Eng. Data 31, 56–70 (1986)
Pirahmadi, F., Dehghani, M.R., Behzadi, B.: Experimental and theoretical study on liquid–liquid equilibrium of 1-butanol + water + NH4Cl at 298.15, 308.15 and 318.15 K. Fluid Phase Equilib. 325, 1–5 (2012)
Sørensen, J.M., Arlt, W.: Liquid–liquid equilibrium data collection. Vol. V, Part 1. DECHEMA, Frankfurt/Main (1980)
Prausnitz, J.M., Anderson, T.F., Grens, E.A., Eckert, C.A., Hsieh, R., O’Connell, J.P.: Computer Calculations for Multicomponent Vapor-Liquid and Liquid–Liquid Equilibria. Prentice Hall, Englewood Cliffs (1980)
Orchillés, A.V., Miguel, P.J., Vercher, E., Martínez-Andreu, A.: Isobaric vapor–liquid equilibria for methyl acetate + methanol + 1-ethyl-3-methylimidazolium trifluoromethanesulfonate at 100 kPa. J. Chem. Eng. Data 52, 915–920 (2007)
Lepori, L., Matteoli, E., Bernazzani, L., Ceccanti, N., Conti, G., Gianni, P., Mollica, V., Tinè, M.R.: Isothermal vapour/liquid equilibria of binary mixtures with dibutyl ether at 298.15 K. Phys. Chem. Chem. Phys. 2, 4837–4842 (2000)
Chen, Y., Wang, H., Tang, Y.Y., Zeng, J.: Ternary (liquid + liquid) equilibria for (water + 2-propanol + α-pinene, or β-pinene) mixtures at four temperatures. J. Chem. Thermodyn. 51, 144–149 (2012)
Nelder, J.A., Mead, R.A.: simplex method for function minimization. Comput. J. 7, 308–313 (1965)
Funding
The authors thank the financial support from National Scientific Research Found of China (21271088).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, W., Zhang, Q. & Chen, Y. Liquid–Liquid Equilibrium of Water + 1-Propanol or 1-Butanol + Dibutyl Ether Ternary Systems: Measurements and Correlation at Three Temperatures. J Solution Chem 47, 498–510 (2018). https://doi.org/10.1007/s10953-018-0733-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-018-0733-0