Abstract
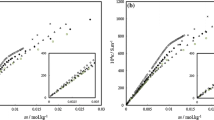
Measurements of the conductance of binary mixtures of cetyltrimethylammonium bromide and sodium dodecylsulfate in pure water and in methanol–water mixed solvent media containing 0.10, 0.20, and 0.30 volume fractions of methanol at 308.15, 318.15, and 323.15 K are reported. The concentration of sodium dodecylsulfate varied from 0.001 to 0.04 mol·L−1 in the presence of ~5.0 × 10−4 mol·L−1 cetyltrimethylammonium bromide. The results showed a sharp increase in the conductance with increasing concentrations of the surfactant mixture. The conductance is found to increase with increasing temperature over the entire concentration range in pure water and in a given mixed solvent medium but is found to decrease with increasing methanol content in the solvent. Estimation of the pre-cmc (S 1) and post-cmc (S 2) slopes for the CTAB–SDS system, to calculate the critical micelle concentration, provides important insight regarding the solution behavior of the mixed surfactants. The critical micelle concentration (cmc) and degree of micellar dissociation (α) of sodium dodecylsulfate in the presence of cetyltrimethylammonium bromide increase in the methanol–water mixed solvent medium. Additionally, the values of cmc and α increase with increasing temperature. The thermodynamic functions for the micellization were calculated at various conditions.









Similar content being viewed by others
References
Tadros, T.F.: Applied Surfactants: Principles and Applications. Wiley, New York (2005)
Holmberg, K., Jönsson, B., Kronberg, B., Lindman, B.: Surfactants and Polymers in Aqueous Solution, 2nd edn. Wiley, Chichester (2003)
Lucassen-Reyolds, E.H., Lucassen, J., Giles, D.: Surface and bulk properties of mixed anionic/cationic surfactant systems. J. Colloid Interface Sci. 81, 150–157 (1981)
Cui, Z.G., Canselier, J.P.: Interfacial and aggregation properties of some anionic/cationic surfactant binary systems II. Mixed micelle formation and surface tension reduction effectiveness. Colloid Polym. Sci. 279, 259–267 (2001)
Wang, C., Lucy, C.A.: Mixed cationic/anionic surfactants for semipermanent wall coatings in capillary electrophoresis. Electrophoresis 25, 825–832 (2004)
Kume, G., Gallotti, M., Nunes, G.: Review on anionic/cationic surfactant mixtures. J. Surfact. Deterg. 11, 1–11 (2008)
Filipović-Vinceković, N., Bujan, M., Dragčević, Đ., Nekić, N.: Phase behavior in mixtures of cationic and anionic surfactants in aqueous solutions. Colloid Polym. Sci. 273, 182–188 (1995)
Segota, S., Tezak, D.: Spontaneous formation of vesicles. Adv. Colloid Interface Sci. 121, 51–75 (2006)
Safran, S.A., Pincus, P., Andelman, D.: Theory of spontaneous vesicle formation in surfactant mixtures. Science 248, 354–356 (1990)
Panda, A.K., Possmayer, F., Petersen, N.O., Nag, K., Moulik, S.P.: Physico-chemical studies on mixed oppositely charged surfactants: their uses in the preparation of surfactant ion selective membrane and monolayer behavior at the air water interface. Colloid Surf A: Physicochem. Eng. Aspects 264, 106–113 (2005)
Herrington, K.L., Kaler, E.W., Miller, D.D., Zasadzinski, J.A., Chiruvolu, S.: Phase behavior of aqueous mixtures of dodecyltrimethylammonium bromide (DTAB) and sodiumdodecyl sulfate (SDS). J. Phys. Chem. 97, 13792–13802 (1993)
Hines, J.D.: Theoretical aspects of micellisation in surfactant mixtures. Curr. Opin. Colloid Interface Sci. 6, 350–356 (2001)
Ogino, K., Abe, M. (eds.): Mixed Surfactant Systems, Surfactant Science Series, vol. 46, pp. 317. Marcel Dekker, New York (1993)
Kronberg, B.: Surfactant mixtures. Curr. Opin. Colloid Interface Sci. 2, 456–463 (1997)
Desai, T.R., Dixit, S.G.: Interaction and viscous properties of aqueous solutions of mixed cationic and nonionic surfactants. J. Colloid Interface Sci. 177, 471–477 (1996)
Shiloach, A., Blankschtein, D.: Predicting micellar solution properties of binary surfactant mixtures. Langmuir 14, 1618–1636 (1998)
Guo, H., Liu, Z., Yang, S., Sun, C.: The feasibility of enhanced soil washing of p-nitrochlorobenzene (pNCB) with SDBS/Tween80 mixed surfactants. J. Haz. Mater. 170, 1236–1241 (2009)
Devinsky, P.M., Lacko, F.I.: Critical micelle concentration, ionization degree and micellisation energy of cationic dimeric (gemini) surfactants in aqueous solution and in mixed micelles with anionic surfactant. Acta Facult. Pharm. Univ. Comenianae 50, 119–131 (2003)
Zana, R., Levy, H., Dganino, D., Talmon, Y., Kwetkat, K.: Mixed micellization of cetyltrimethylammonium bromide and an anionic dimeric (gemini) surfactant in aqueous solution. Langmuir 13, 402–408 (1997)
Schulz, P.C., Minardi, R.M., Vuano, B.: Dodecyltrimethylammonium bromide–disodium dodecanephosphate mixed micelles. Colloid Polym. Sci. 277, 837–845 (1999)
Patist, A., Chhabra, V., Pagidipati, R., Shah, R., Shah, D.O.: Effect of chain length compatibility on micellar stability in sodium dodecylsulphate/alkyltrimethylammonium bromide solutions. Langmuir 13, 432–434 (1997)
Kato, T., Takeuchi, H., Seimiya, T.: Concentration dependence of micellar size and composition in mixed anionic/cationic surfactant solutions studied by light scattering and pulsed-gradient FT–NMR spectroscopy. J. Phys. Chem. 96, 6839–6843 (1992)
Xiao, J.X., Bao, Y.X.: An unusual variation of surface tension with concentration of mixed cationic–anionic surfactants. Chin. J. Chem. 19, 73–75 (2001)
Rodriguez, J., Clavero, E., Laria, D.: Computer simulations of catanionic surfactants adsorbed at air/water interfaces. J. Phys. Chem. B 109, 24427–24433 (2005)
Sohrabi, B., Gharibi, H., Tajik, B., Javadian, S., Hashemianzadeh, M.: Molecular interactions of cationic and anionic surfactants in mixed monolayers and aggregates. J. Phys. Chem. B 112, 14869–14876 (2008)
Mukerjee, P., Mysels, K.J.: Critical Micelle Concentration of Aqueous Surfactant Systems. National Bureau of Standards, Washington DC (1970)
Khan, A.M., Shah, S.S.: Determination of critical micelle concentration of sodium dodecylsulphate and effect of low concentration pyrene on its cmc using origin software. J. Chem. Soc. Pak. 30, 186–191 (2008)
Cifuentes, A., Bernal, J.L., Deiz-Masa, J.C.: Determination of critical micelle concentration values using capillary electrophoresis instrumentation. Anal. Chem. 69, 4271–4274 (1997)
Tang, Y., Du, B.Y., Yang, J., Zhang, Y.M.: Temperature effects on surface activity and application in oxidation of toluene derivatives of CTAB–SDS with KMnO4. J. Chem. Sci. 118, 281–285 (2006)
Jenkins, S.I.: Novel Aqueous Two-Phase Systems: Characterization and Applications in Chemical Separation. Ph.D. dissertation, North Carolina State University (2012)
Huang, J.B., Zhu, B.Y., Zhao, G.X., Zhang, Z.Y.: Vesicle formation of a 1:1 catanionic surfactant mixture in ethanol solution. Langmuir 13, 5759–5761 (1997)
Zana, R., Michels, B.: On the formation of vesicles by mixtures of anionic and cationic surfactants in ethanol. Langmuir 14, 6599–6602 (1998)
Huang, J.-B., Zhu, B.-Y., Mao, M., He, P., Wang, J., He, X.: Vesicle formation of 1:1 cationic and anionic surfactant mixtures in nonaqueous polar solvents. Colloid Polym. Sci. 277, 354–360 (1999)
Tomasic, V., Stefanic, I., Filipovic-Vincekovic, N.: Adsorption, association and precipitation in hexadecyltrimethylammonium bromide/sodium dodecylsulphate mixtures. Colloid Polym. Sci. 277, 153–163 (1999)
Bhattarai, A., Chatterjee, S.K., Niraula, T.P.: Effects of concentration, temperature and solvent composition on density and apparent molar volume of the binary mixtures of cationic–anionic surfactants in methanol–water mixed solvent media. SpringerPlus 2, 280 (2013)
Apelblat, A.: Representation of electrical conductances for polyvalent electrolytes by the Quint–Viallard conductivity equation. Part 4. Symmetrical 2:2, 3:3 and unsymmetrical 2:1, 3:1 and 1:3 type electrolytes in pure organic solvents. J. Solution Chem. 40, 1234–1257 (2011)
Bhattarai, A., Nandi, P., Das, B.: The effects of concentration, relative permittivity and temperature on the transport properties of sodium polystyrenesulphonate in methanol–water mixed solvent media. J. Polym. Res. 13, 475–482 (2006)
Bhattarai, A., Chatterjee, S.K., Deo, T.K., Niraula, T.P.: Effects of concentration, temperature, and solvent composition on the partial molar volumes of sodium lauryl sulfate in methanol (1) + water (2) mixed solvent media. J. Chem. Eng. Data 56, 3400–3405 (2011)
Chatterjee, A., Das, B.: Electrical conductances of tetrabutylammonium bromide, sodium tetraphenylborate, and sodium bromide in methanol (1) + water (2) mixtures at (298.15, 308.15, and 318.15) K. J. Chem. Eng. Data 51, 1352–1355 (2006)
Albright, P.S., Gasting, L.J.: Dielectric constants of methanol–water system from 5 to 55°. J. Am. Chem. Soc. 68, 1061–1063 (1946)
Yilmaz, H., Guler, S.: Excess properties of methanol–water binary system at various temperatures. Il Nuovo Cimento D 20, 1853–1861 (1998)
Chakraborty, T., Chakraborty, I., Ghosh, S.: Sodium carboxymethylcellulose–CTAB interaction: a detailed thermodynamic study of polymer–surfactant interaction with opposite charges. Langmuir 22, 9905–9913 (2006)
Lin, S.Y., Lin, Y.Y., Chen, E.M., Hsu, C.T., Kwan, C.C.: A study of the equilibrium surface tension and the critical micelle concentration of mixed surfactant solutions. Langmuir 15, 4370–4376 (1999)
Umlong, I.M., Ismail, K.: Micellization behaviour of sodium dodecylsulphate in different electrolyte media. Colloids and Surfaces A: Physicochem. Eng. Aspects 299, 8–14 (2007)
Lind Jr, J.E., Zwolenik, J.J., Fuoss, R.M.: Calibration of conductance cells at 25 °C with aqueous solutions of potassium chloride. J. Am. Chem. Soc. 81, 1557–1559 (1957)
Das, B., Hazra, D.K.: Studies on the viscosities, conductance and adiabatic compressibilities of some tetraalkylammonium perchlorates in 2-methoxyethanol. Bull. Chem. Soc. Jpn. 65, 3470–3476 (1992)
Das, B., Hazra, D.K.: Conductometric, viscometric, and spectroscopic investigations on the solvation phenomena of alkali-metal ions and ion pairs in 2-methoxyethanol. J. Phys. Chem. 99, 269–273 (1995)
Zana, R.: Ionization of cationic micelles: effect of the detergent structure. J. Colloid Interface Sci. 78, 330–337 (1980)
Aslanzadeh, S., Yousefi, A.: The effect on ethanol on nanostructures of mixed cationic and anionic surfactants. J. Surfactant Deterg. 17, 709–716 (2014)
del Rio, J.M., Prieto, G., Sarmiento, F., Mosquera, V.: Thermodynamics of micellization of N-octyltrimethylammonium bromide in different media. Langmuir 11, 1511–1514 (1995)
Attwood, D., Florence, A.T.: Surfactant Systems: Their Chemistry, Pharmacy and Biology. Chapman and Hall, London (1983)
Khan, A., Marques, E.: Catanionic Surfactants in Specialists Surfactants. Blackie Academic and Professional, pp. 37–76. Chapman and Hall, London (1997)
Chen, L., Xiao, J.-X., Ruan, K., Ma, J.: Homogeneous solutions of equimolar mixed cationic–anionic surfactants. Langmuir 18, 7250–7252 (2002)
Bakshi, M.S.: Mixed micelles of cationic surfactants in aqueous polyethylene glycol 1000. J. Dispers. Sci. Technol. 20, 1715–1735 (1999)
Bakshi, M.S.: Cetylpyridinium chloride + tetradecyltrimethylammonium bromide mixed micelles in polyethylene glycol 1000 + water mixtures. J. Macromol. Sci. Pure Appl. Chem. A 36, 879–892 (1999)
Javadian, S., Gharibi, H., Sohrabi, B., Bijanzadeh, H., Safarpour, M.A., Behjatmanesh, A.: Determination of the physico-chemical parameters and aggregation number of surfactant in micelles in binary alcohol–water mixtures. J. Mol. Liq. 137, 74–79 (2008)
Varade, D., Joshi, T., Aswal, V.K., Goyal, P.S., Hassand, P.A., Bahadur, P.: Effect of salt on the micelles of cetylpyiridinium chloride. Colloid Surf. A: Physicochem. Eng. Aspects 259, 95–101 (2005)
Dubey, N.: A conductometric study of interaction between sodium dodecyl sulfate and 1-propanol, 1-butanol 1-pentanol and 1-hexanol at different temperatures. J. Surface Sci. Technol. 24, 139–148 (2008)
Hiemenz, P.C., Rajagopalan, R.: Principles of Colloid and Surface Chemistry, pp. 335–404. Dekker, New York (1997)
Norvaisas, P., Petrauskas, V., Matulis, D.: Thermodynamics of cationic and anionic surfactant interaction. J. Phys. Chem. B 116, 2138–2144 (2012)
Rub, M.A., Asiri, A.M., Naqvi, A.Z., Rahman, M.M., Khan, S.B., Din, K.: Mixed micellization between amphiphilic drug promethazine hydrochloride and cationic surfactant(conventional as well as gemini). J. Mol. Liq. 177, 19–25 (2013)
Talhout, R., Engberts, J.: Self-Assembly in mixtures of sodium alkyl sulfates and alkyltrimethylammonuium bromides: aggregation behavior and catalytic properties. Langmuir 13, 5001–5006 (1997)
Li, W., Han, Y.C., Zhang, J.L., Wang, L.X., Song, J.: Thermodynamic modeling of CTAB aggregation in water–ethanol mixed solvents. Colloid J. 68, 304–310 (2006)
Moulik, S.P., Haque, M.E., Jana, P.K., Das, A.R.: Micellar properties of cationic surfactants in pure and mixed states. J. Phys. Chem. 100, 701–708 (1996)
Mitra, D., Chakraborty, I., Bhattacharya, S.C., Moulik, S.P.: Interfacial and solution properties of tetraalkylammonium bromides and their sodiumdodecyl sulfate interacted products: a detailed physicochemical study. Langmuir 23, 3049–3061 (2007)
Acknowledgments
This work was supported by a Research Grant for the fiscal year 2012/2013 of Nepal Academy of Science and Technology (NAST), Khumaltar, Lalitpur, Nepal under Research Grant Program. This work was also supported by the grant from University Grants Commission, Nepal for some chemicals. The author is grateful to Professor Bijan Das, presently in the Department of Chemistry, Presidency University, Kolkata, India for valuable suggestions and discussions. The author sincerely thanks the Head of the Department of Chemistry, Mahendra Morang Adarsh Multiple Campus, Tribhuvan University, Biratnagar, Nepal for providing the available research facilities to conduct this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhattarai, A. Studies of the Micellization of Cationic–Anionic Surfactant Systems in Water and Methanol–Water Mixed Solvents. J Solution Chem 44, 2090–2105 (2015). https://doi.org/10.1007/s10953-015-0391-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-015-0391-4