Abstract
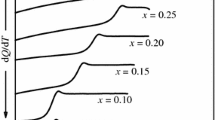
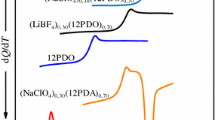
Dielectric behavior and glass formation in supercooled liquid guaifenesin (GFN) and its binary liquids with acetaminophen (ACT), and of ACT with mephenesin (MP), are reported using dielectric spectroscopy (10−3 Hz–2 MHz) and differential scanning calorimetry down to liquid nitrogen temperature. Solid–liquid phase equilibria of ACT + GFN and ACT + MP exhibit a simple eutectic of complete miscibility in the liquid state with eutectic points at 344.6 (±1) K and 337.5 (±1.4) K, respectively, and separate into two crystalline components in the solid state. The glass transition temperature (T g) measured for quenched binary liquids obeys a mixture rule. The primary relaxation process (α-process), can be well described by the Havriliak–Negami equation. A secondary relaxation process (β-process), found below T g, is Arrhenius in its temperature dependence, but may not be of inter-molecular or Johari–Goldstein (JG) type in nature. The “fragility” of the samples is discussed in the context of a coupling model. The supercooled liquid samples and binary liquids studied here are found to be “very fragile” in nature.













Similar content being viewed by others
References
Wang, J., Angell, C.A.: Glass Structure by Spectroscopy. Marcel Dekkar, New York (1976)
Johari, G.P., Goldstein, M.: Viscous liquids and the glass transition III. Secondary relaxations in aliphatic alcohols and other non-rigid molecules. J. Chem. Phys. 55, 4245–4252 (1971)
Johari, G.P., Goldstein, M.: Viscous liquids and the glass transition II. Secondary relaxations in glasses of rigid molecules. J. Chem. Phys. 53, 2372–2388 (1970)
Okamoto, N., Oguni, M.: Discovery of crystal nucleation preceding much below the glass transition temperature in a supercooled liquid. Solid State Commun. 99, 53–56 (1996)
Hikima, T., Hanaya, M., Oguni, M.: β-Molecular rearrangement process, but not an α-process, as governing the homogeneous crystal-nucleation rate in a supercooled liquid. Bull. Chem. Soc. Jpn. 69, 1863–1868 (1996)
Hikima, T., Hanaya, M., Oguni, M.: Microscopic observation of a peculiar crystallization in the glass transition region and β-process as potentially controlling the growth rate in triphenylethylene. J. Mol. Struct. 479, 245–250 (1999)
Paladi, F., Oguni, M.: Anomalous generation and extinction of crystal nuclei in nonequilibrium supercooled liquid o-benzylphenol. Phys. Rev. B. 65, 144202–144208 (2002)
Paladi, F., Oguni, M.: Generation and extinction of crystal nuclei in an extremely nonequilibrium glassy state of salol. J. Phys. Condens. Matter. 15, 3909–3917 (2003)
Hatase, M., Hanaya, M., Hikima, T., Oguni, M.: Discovery of homogeneous-nucleation-based crystallization in simple glass-forming liquid of toluene below its glass-transition temperature. J. Non-Cryst. Solids 307–310, 257–263 (2002)
Murthy, S.S.N., Nayak, S.K.: Experimental study of the nature of the glass transition process in monohydroxy alcohols. J. Chem. Phys. 99, 5362–5368 (1983)
Johari, G.P.: Glass transition and secondary relaxations in molecular liquids and crystals. Ann. New York Acad. Sci. 279, 117–140 (1976)
Reid, C.J., Evans, M.W.: Dielectric and far infrared study of solutions in the glassy state from 100 Hz to 10 THz: discovery and characterization of the universal γ process. J. Chem. Phys. 76, 2576–2584 (1982)
Wu, L., Nagel, S.R.: Secondary relaxation in o-terphenyl glass. Phys. Rev. D. 46, 11198–11200 (1992)
Gangasharan, Murthy, S.S.N.: Study of α-, β-, and γ-relaxation processes in some supercooled liquids and supercooled plastic crystals. J. Chem. Phys. 99, 9865–9873 (1993)
Shahin, Md, Murthy, S.S.N.: Sub-T g relaxations due to dipolar solutes in nonpolar glass-forming solvents. J. Chem. Phys. 122, 014507 (15) (2005)
Johari, G.P., Kim, S., Shanker, R.M.: Dielectric relaxation and crystallization of ultraviscous melt and glassy states of aspirin, ibuprofen, progesterone, and quinidine. J. Pharm. Sci. 96, 1159–1175 (2007)
Barro, M., Espeau, P., Tamarit, J.L., Perrin, M.A., Veglio, N., Ceolin, R.: Polymorphism of progesterone: relative stabilities of the orthorhombic phases I and II inferred from topological and experimental pressure temperature phase diagrams. J. Pharm. Sci. 98, 1657–1670 (2009)
Kerc, J., Srcic, S., Mohar, M., Korbar, J.S.: Some physicochemical properties of glassy felodipine. Int. J. Pharm. 68, 25–33 (1991)
Sailaja, U., Thayyil, M.S., Kumar, N.S.K., Govindaraj, G.: Molecular dynamics in liquid and glassy states of non-steroidal anti-inflammatory drug: ketoprofen. Eur. J. Pharm. Sci. 49, 333–340 (2013)
Mahe, N., Perrin, M.A., Barrio, M., Nicolai, B., Rietveld, I.B., Tamarit, J.L., Ceolin, R.: Solid state studies of the triclinic (Z = 2) antiprotozoal drug ternidazole. J. Pharm. Sci. 100, 2258–2266 (2011)
Mooter, G.V., Augustijins, P., Kinget, R.: Stability prediction of amorphous benzodiazepines by calculation of the mean relaxation time constant using the Williams Watts decay function. Eur. J. Pharm. Biopharm. 48, 43–48 (1999)
Johari, G.P., Kim, S., Shanker, R.M.: Dielectric studies of molecular motions in amorphous solid and ultraviscous acetaminophen. J. Pharm. Sci. 94, 2207–2223 (2005)
Espeau, P., Ceolin, R., Tamarit, J.L., Perrin, M.A., Gauchi, J.P., Leveiller, F.: Polymorphism of paracetamol: relative stabilities of the monoclinic and orthorhombic phase inferred from topological pressure temperature and temperature volume phase diagram. J. Pharm. Sci. 94, 524–539 (2005)
Rengarajan, G.T., Beiner, M.: Relaxation behaviour and crystallization kinetics of amorphous acetaminophen. Lett. Drug Design Discov. 3, 723–730 (2006)
Saini, M.K., Murthy, S.S.N.: Study of glass transition phenomenon in the supercooled liquid phase of methocarbamol, acetaminophen and mephenesin. Thermchim. Acta 575, 195–205 (2014)
Johari, G.P., Kim, S., Shanker, R.M.: Dielectric study of equimolar acetaminophen–aspirin, acetaminophen–quinidine, and benzoic acid–progesterone molecular alloys in the glass and ultraviscous states and their relevance to solubility and stability. J. Pharm. Sci. 99, 1358–1374 (2010)
Stott, P.W., Williams, A.C., Barry, B.W.: Transdermal delivery from eutectic systems: enhanced permeation of a model drug, ibuprofen. J. Control. Release 50, 297–308 (1998)
Tu, W., Chen, Z., Gao, Y., Li, Z., Zhang, Y., Liu, R., Tian, Y., Wang, L.M.: Glass transition mixing thermodynamics of a binary eutectic system. Phys. Chem. Chem. Phys. 16, 3586–3592 (2014)
Liu, D., Fei, X., Wang, S., Jiang, T., Su, D.: Increasing solubility and dissolution rate of drugs via eutectic mixture: itraconazole–poloxamer 188 system. Asian J. Pharm. Sci. 1, 213–221 (2006)
Gala, U., Pham, H., Chauhan, H.: Pharmaceutical applications of eutectic mixtures. J. Dev. Drugs. (2013). doi:10.4172/2329-6631.1000e130
McCrum, N.G., Read, D.E., Williams, G.: Inelastic and Dielectric Effects in Polymeric Solids. Wiley, New York (1967)
Jonscher, A. K.: Dielectric Relaxation in Solids. Chelsea, London (1983) (The dielectric loss in the frequency range 10−0.5–10−3 is obtained from the Hamon’s approximation: ε″(f) = i(t) * t/0.63C 0 V 0 with ft = 0.l, where i(t) is the discharging current at a time t, C 0 is the empty cell capacitance, and V 0 is the applied voltage)
Hamon, B.V.: An approximation method for deducing dielectric loss factor from direct-current measurements. Inst. Monogr. 99, 151–155 (1952)
Kita, Y., Koizumi, N.: Remarks on the Hamon approximation. Adv. Mol. Relax. Process. 7, 13–20 (1975)
Bredikhin, A.A., Gubaidullin, A.T., Bredikhina, Z.A., Krivolapov, D.B., Pashagin, A.V., Litvinov, I.A.: Absolute configuration and crystal packing for three chiral drugs prone to spontaneous resolution: guaifenesin, methocarbamol and mephenesin. J. Mol. Struct. 920, 377–382 (2009)
Brittain, H.G.: Analytic Profiles of Drug Substances and Excipients, vol. 25, pp. 121–164. Acute Therapeutics, Inc, New Jersey (1998)
Havriliak, S., Negami, S.: A complex plane analysis of α-dispersions in some polymer systems. J. Polym. Sci. Part C. Polym. Symp. 14, 99–117 (1966)
Murthy, S. S. N.: Phase behavior of the supercooled aqueous solutions of dimethyl sulfoxide, ethylene glycol, and methanol as seen by dielectric spectroscopy, J. Phys. Chem. B. 101, 6043–6049 (1997) {The exact relation between f m and f 0 is f m = f 0 {k′/[cos(απ/2) − sin(απ/2).k′]}1/(1 –α) where k′ = tan [(1–α)π/(2(1 + β))]}
Murthy, S.S.N., Kumar, D.: Glass formation in organic binary liquids studied using differential scanning calorimetry. J. Chem. Soc. Faraday Trans. 89, 2423–2427 (1993)
Murthy, S.S.N.: Temperature dependence of the dielectric relaxation process in glass-forming materials. J. Chem. Soc. Faraday Trans. 84, 671–677 (1988)
Hill, N.E., Vaughan, W.E., Price, A.H., Davies, M.: Dielectric Properties and Molecular Behaviour. Van Nostrand Reinhold, London (1969)
Angell, C.A.: Relaxation in liquids, polymers and plastic crystals—strong/fragile patterns and problems. J. Non-Cryst. Solids 131–133, 13–31 (1991)
Zhou, D., Zhang, G.G.Z., Law, D., Grant, D.J.W., Schmitt, E.A.: Physical stability of amorphous pharmaceuticals. Importance of configurational thermodynamic quantities and molecular mobility. J. Pharm. Sci. 91, 1863–1872 (2002)
Espeau, P., Ceolin, R., Tamarit, J.L., Perrin, M.A., Gauchi, J.P., Leveiller, F.: Polymorphism of paracetamol. Relative stabilities of the monoclinic and orthorhombic phases inferred from topological pressure–temperature and temperature–volume phase diagrams. J. Pharm. Sci. 94, 524–539 (2005)
Martino, P.D., Palmieri, G.F., Martelli, S.: Molecular mobility of the paracetamol amorphous form. Chem. Pharm. Bull. 48, 1105–1108 (2000)
Klimova, K., Leitner, J.: DSC study and phase diagrams calculation of binary systems of paracetamol. Thermochim. Acta 550, 59–64 (2012)
Capacioli, S., Nagai, K.L.: Relation between the α-relaxation and Johari–Goldstein β-relaxation of a component in binary miscible mixtures of glass-formers. J. Phys. Chem. B. 109, 9727–9735 (2005)
Ngai, K.L., Capacioli, S.: Relation between the activation energy of the Johari–Goldstein β-relaxation and Tg of glass formers. Phys. Rev. E. 69, 031501 (5) (2004)
Alvarez, F., Alegria, A., Colmenero, J.: Relationship between the time-domain Kohlrausch–Williams–Watts and frequency-domain Havriliak–Negami relaxation functions. Phys. Rev. B., Condens. Matter. 44, 7306–7312 (1991)
Murthy, S.S.N., Gangasharan, Nayak, S.K.: Noval differential scanning calorimetric studies of supercooled organic liquids. J. Chem. Soc. Faraday Trans. 89, 509–514 (1993)
Rodrigues, A.C., Viciosa, M.T., Danede, F., Affouard, F., Correia, N.T.: Molecular mobility of amorphous S-flurbiprofen: a dielectric relaxation spectroscopy approach. Mol. Pharm. 11, 112–130 (2013)
Wang, L.M., Angell, C.A., Richert, R.: Fragility and thermodynamic in nonpolymeric glass-forming liquids. J. Chem. Phys. 125, 074505–074513 (2006)
Wang, L.M., Angell, C.A.: Response to comment on direct determination of the fragility indices of glass forming liquids by differential scanning calorimetry: kinetic versus thermodynamic fragilities. J. Chem. Phys. 118, 10353–10355 (2003)
Bohmer, R., Ngai, K.L., Angell, C.A., Plazek, D.J.: Nonexponential relaxations in strong and fragile glass formers. J. Chem. Phys. 99, 4201–4209 (1993)
Shamblin, S.L., Hancock, B.C., Dupuis, Y., Pikal, M.J.: Interpretation of relaxation time constants for amorphous pharmaceutical systems. J. Pharm. Sci. 89, 417–427 (2000)
Ngai, K.L.: Relation between some secondary relaxations and the α relaxations in glass-forming materials according to the coupling model. J. Chem. Phys. 109, 6982–6994 (1998)
Colby, R.H.: Dynamic scaling approach to glass formation. Phys. Rev. E 61, 1783–1792 (2000)
Drozd-Rzoska, A., Rzoska, S.J., Pawlus, S., Garcia, M., Tammarit, J.L.: Evidence for critical like behavior in ultra-slowing glass forming systems. Phys. Rev. E 82, 031501–031509 (2010)
Martinez Garcia, J.C., Tamrit, J.L., Rzoska, S.J.: Enthalpy space analysis of the evolution of the primary relaxation time in ultra-slowing system. J. Chem. Phys. 134, 024512–024519 (2011)
Acknowledgments
The authors like to thank Dept. of Science & Technology & UGC, Govt. of India for the financial support. One of the authors (Manoj K. Saini) acknowledges the Research fellowship from UGC, India.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saini, M.K., Murthy, S.S.N. Glass Formation in Binary Solutions of Acetaminophen with Guaifenesin and Mephenesin. J Solution Chem 44, 1723–1748 (2015). https://doi.org/10.1007/s10953-015-0364-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-015-0364-7