Abstract
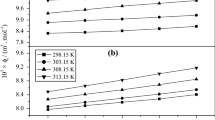
Values of the critical micelle concentration (cmc) and degree of counterion dissociation (α) of four cationic surfactants: cetyltrimethylammonium bromide (CTAB), cetylpyridinium bromide (CPB), cetylpyridinium chloride (CPC) and benzyldimethylhexadecylammonium chloride (BDHAC) in aqueous–glycine medium (concentration of glycine (Gly) varied from 0 to 0.20 mol·dm−3) were determined through conductometric measurements at 303 K. The effect of temperature on the micellization in the presence of 0.10 mol·dm−3 Gly were studied for the surfactants CTAB, CPC and BDHAC. With respect to the concentration of Gly, a decrease in the cmc was observed for CTAB, CPC and CPB whereas an increase was observed for BDHAC. A regular increase in α was obtained for CPB, CPC and BDHAC with respect to the concentration of Gly whereas values were roughly constant in the case of CTAB. Thermodynamic parameters were computed from the temperature dependence of the cmc values and it was found that the micellization process is exothermic. Compensation of enthalpy and entropy was observed for the micellization of CTAB, CPC and BDHAC in the presence of 0.10 mol·dm−3 Gly.







Similar content being viewed by others
References
Rosen, M.J.: Surfactants and Interfacial Phenomena. Wiley, New York (2004)
Haldar, J., Aswal, V.K., Goyal, P.S., Bhattacharya, S.: Role of incorporation of multiple head groups in cationic surfactants in determining micellar properties. Small angle neutron scattering and fluorescence studies. J. Phys. Chem. B 105, 12803–12808 (2001)
Atwood, D., Florence, A.T.: Surfactant Systems: Their Chemistry, Pharmacy and Biology. Chapman & Hall, London (1983)
David S.L., Kumar S., Kabir-ud-Din: Viscosities of cetylpyridinium bromide solutions (aqueous and aqueous KBr) in the presence of alcohols and amines. J. Chem. Eng. Data 42, 198–201 (1997)
Sulthana, S.B., Rao, P.V.C., Bhat, S.G.T., Rakshit, A.K.: Interfacial and thermodynamic properties of SDBS–C12E10 mixed micelles in aqueous media: effect of additives. J. Phys. Chem. B 102, 9653–9660 (1998)
Chakraborty, T., Ghosh, S., Moulik, S.P.: Micellization and related behavior of binary and ternary surfactant mixtures in aqueous medium: cetyl pyridinium chloride (CPC), cetyl trimethyl ammonium bromide (CTAB), and polyoxyethylene (10) cetyl ether (Brij-56) derived system. J. Phys. Chem. B 109, 14813–14823 (2005)
Ray, G.B., Chakraborty, I., Ghosh, S., Moulik, S.P.: Studies on binary and ternary amphiphile combinations of tetradecyltrimethylammonium bromide (C14TAB), tetradecyltriphenylphosphonium bromide (C14TPB), and tetradecylpyridinium bromide (C14PB). A critical analysis of their interfacial and bulk behaviours. J. Phys. Chem. B 111, 9828–9837 (2007)
Das, D., Ismail, K.: Aggregation and adsorption properties of sodium dodecyl sulfate in water–acetamide mixtures. J. Coll. Interface Sci. 327, 198–203 (2008)
Lehninger, A.L., Nelson, D.L., Cox, M.M.: Principles of Biochemistry. Worth Publishers, USA (1993)
Sharma, K.S., Hassan, P.A., Rakshit, A.K.: Surface activity and association behavior of nonaoxyethylene n-dodecylether in aquo amino acid medium: tensiometry, small-angle neutron scattering, dynamic light scattering and viscosity studies. Colloids Surf. A 308, 100–110 (2007)
Leodidis, E.B., Hatton, T.A.: Amino acids in AOT reversed micelles. 1. Determination of interfacial partition coefficients using the phase transfer method. J. Phys. Chem. 94, 6411–6420 (1990)
Leodidis, E.B., Bommarius, A.S., Hatton, T.A.: Amino acids in reversed micelles. 3. Dependence of the interfacial partition coefficient on excess phase salinity and interfacial curvature. J. Phys. Chem. 95, 5943–5956 (1991)
Adachi, M., Harada, M., Shioi, A., Sato, Y.: Extraction of aminoacids to microemulsion. J. Phys. Chem. 95, 7925–7931 (1991)
Adamson, A.W.: Physical Chemistry of Surfaces. John-Wiley, New York (1976)
Luethi, P., Luisi, P.L.: Enzymatic synthesis of hydrocarbon-soluble peptides with reverse micelles. J. Am. Chem. Soc. 106, 7285–7286 (1984)
Ruiz, C.C., M.-Bolivar, J.A., Aguiar, J., P.-Garcia, J.M.: Aggregation behaviour of octyl-β-thioglucopyranoside in the presence of glycine. Colloids Surf. A 249, 35–39 (2004)
Pandey, E., Upadhyay, S.K.: Effect of micellar aggregates on the kinetics of oxidation of α-aminoacids by chloramine-T in perchloric acid medium. Colloids Surf. A 269, 7–15 (2005)
Shukla, R., Upadhyay, S.K.: Tween-80 catalysis in the oxidation of methionine and proline by alkaline hexacyanoferrate(III). Colloids Surf. A 331, 245–249 (2008)
Harutyunyana, N.G., Harutyunyana, L.R., Harutyunyan, R.S.: Volumetric properties of amino acids in aqueous solution of nonionic surfactant. Thermochim. Acta 498, 124–127 (2010)
Ali, A., Tariq, M., Patel, R., Ittoo, F.A.: Interaction of glycine with cationic, anionic, and nonionic surfactants at different temperatures: a volumetric, viscometric, refractive index, conductometric, and fluorescence probe study. Colloid Polym. Sci. 286, 183–190 (2008)
Ruiz, C.C., Hierrezuelo, J.M., M.-Bolivar, J.A.: Effect of glycine on the surface activity and micellar properties of N-decanoyl-N-methylglucamide. Colloid Polym. Sci. 286, 1281–1289 (2008)
Chauhan, S., Sharma, K., Rana, D.S., Kumar, G., Umar, A.: Volumetric and conductance studies of cetyltrimethyl ammonium bromide in aqueous glycine. J. Solution Chem. 42, 634–656 (2013)
Yan, Z., Wang, J., Kong, W., Lu, J.: Effect of temperature on volumetric and viscosity properties of some α-amino acids in aqueous calcium chloride solutions. Fluid Phase Equilib. 215, 143–150 (2004)
Badarayani, R., Kumar, A.: Viscometric study of glycine, l-alanine, glycylglycine in aqueous tetra-n-alkylammonium bromide solutions at 298.15 K. J. Chem. Thermodyn. 36, 983–991 (2004)
Singh, S.K., Kundu, A., Kishore, N.: Interactions of some amino acids and glycine peptides with aqueous sodium dodecyl sulfate and cetyltrimethylammonium bromide at T = 298.15 K: a volumetric approach. J. Chem. Thermodyn. 36, 7–16 (2004)
Carpena, P., Aguiar, J., B.–Galvan, P., Ruiz, C.C.: Problems associated with the treatment of conductivity—concentration data in surfactant solutions: simulations and experiments. Langmuir 18, 6054–6058 (2002)
Chauhan, S., Chauhan, M.S., Sharma, P., Rana, D.S.: Thermodynamics and micellization of cetyltrimethyl ammonium bromide in the presence of lysozyme. J. Mol. Liquids 187, 1–6 (2013)
Chauhan, S., Sharma, K., Rana, D.S., Kumar, G., Umar, A.: Conductance, apparent molar volume and compressibility studies of cetyltrimethylammonium bromide in aqueous solution of leucine. J. Mol. Liq. 175, 103–110 (2012)
Tanford, C.: The Hydrophobic Effect: Formation of Micelles and Biological Membranes. Wiley, New York (1980)
Lumry, R., Rajender, S.: Enthalpy–entropy compensation phenomena in water solutions of protein and small molecules: a ubiquitous property of water. Biopolymers 9, 1125–1227 (1970)
Frank, H.S., Evans, M.W.: Free volume and entropy in condensed systems III. Entropy in binary liquid mixtures; Partial molal entropy in dilute solutions; Structure and thermodynamics in aqueous electrolytes. J. Chem. Phys. 13, 507–532 (1945)
Acknowledgments
T. A. W. gratefully acknowledges fellowship received from the University Grants Commission, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koya, P.A., Wagay, T.A. & Ismail, K. Conductometric Studies on Micellization of Cationic Surfactants in the Presence of Glycine. J Solution Chem 44, 100–111 (2015). https://doi.org/10.1007/s10953-014-0284-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-014-0284-y