Abstract
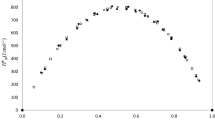
Excess molar enthalpies, ∆H Em , for the binary mixtures of butyl acetate + 1-alkanols, namely (methanol, ethanol, 1-propanol, 1-butanol, 1-pentanol, and 1-hexanol), were measured over the whole range of composition at 298.15 K using a Parr 1455 solution calorimeter. The excess partial molar enthalpies, ∆H Em,i , were calculated from the experimental excess molar enthalpies using the Redlich–Kister polynomial equation. The sign of ∆H Em for all systems are positive because of the disruption of hydrogen bonding and dipole–dipole interactions in the alkanols and esters, respectively. The magnitude of the ∆H Em values increases with increasing alkyl chain length. The behavior of ∆H Em was analyzed in terms of the length of the alkanol chain, the nature and type of intermolecular interactions and the balance between positive and negative effects on deviations from ideality. The experimental excess molar enthalpy data have also been correlated using the Redlich–Kister and SSF equations and two local composition models (UNIQUAC and NRTL).


Similar content being viewed by others
Abbreviations
- A k :
-
Adjustable parameters of Redlich–Kister equation
- A i , B i :
-
Adjustable parameters of SSF equation
- Δu 12, Δu 21 :
-
Adjustable parameters contained in the UNIQUAC model
- Δg 12, Δg 21 :
-
Interaction energy parameters in the NRTL model
- α 12 :
-
Nonrandomness parameter in the NRTL model
- ∆H Em :
-
Excess molar enthalpy
- ∆H Em,i :
-
Excess partial molar enthalpy
- ρ :
-
Density
- n D :
-
Refractive index
- R :
-
Universal gas constant
- T :
-
Kelvin temperature
- x i :
-
Mole fraction of component i
- q :
-
Structural parameters in the UNIQUAC model
- σ:
-
Standard deviation
References
Prausnitz, J.M., Lichtenthaler, R.N., de Azevedo, E.G.: Molecular Thermodynamics of Fluid Phase Equilibria, 3rd edn. Prentice-Hall PTR, Upper Saddle River (1999)
Iloukhani, H., Khanlarzadeh, K.: Volumetric properties for binary and ternary systems consist of 1-chlorobutane, n-butylamine and isobutanol at 298.15 K and ambient pressure with application of the Prigogine–Flory–Patterson theory (PFP) and ERAS-Model. Thermochim. Acta 502, 77–84 (2010)
Khanlarzadeh, K., Iloukhani, H.: Application of ERAS-Model and Prigogine–Flory–Patterson theory to excess molar volumes for ternary mixtures of 2-chlorobutane (1) + butylacetate (2) + isobutanol (3) at T = 298.15 K. J. Chem. Thermodyn. 43, 1583–1590 (2011)
Iloukhani, H., Khanlarzadeh, K.: Physicochemical properties of quaternary systems and comparison of different geometrical models. J. Chem. Eng. Data 11, 4244–4252 (2011)
Iloukhani, H., Zarei, H.A.: Excess molar enthalpies of N, N-dimethylformamide + alkan-1-ols (C1–C6) at 298.15 K. J. Chem. Eng. Data 47, 195–197 (2002)
Iloukhani, H., Fattahi, M.: Correlation of excess molar enthalpies of cyclopentanone (1) + 1-alkanols (C1–C5) (2) by Peng–Robinson–Stryjek–Vera equation of state and ERAS-model. J. Mol. Liq. 171, 37–42 (2012)
Khanlarzadeh, K., Iloukhani, H.: Experimental and theoretical study on excess molar enthalpy of binary mixtures containing isobutanol (1) + alkan-1-ols (C1–C4) (2) at T = 298.15 K. J. Mol. Liq. 187, 24–29 (2013)
Gao, H., Li, S., Yan, W.: Excess molar enthalpies of ethylacetoacetate + (methanol, + ethanol, + 1-propanol, and + 2-propanol) at T = (298.15, 313.15 and 328.15) K, p = (0.1 and 10.0 MPa). Fluid Phase Eqilib. 268, 34–38 (2008)
Bravo-Sanchez, M.G., Iglesias-Silva, G.A., Estrada-Baltazar, A., Hall, K.R.: Densities and viscosities of binary mixtures of n-butanol with 2-butanol, isobutanol, and tert-butanol from (303.15 to 343.15) K. J. Chem. Eng. Data 55, 2310–2315 (2010)
Sheu, Y.W., Tu, C.H.: Densities, viscosities, refractive indices, and surface tensions for 12 flavor esters from T = 288.15 K to T = 358.15 K. J. Chem. Eng. Data 50, 1706–1710 (2005)
Matsuo, H., Tu, C.H., Wong, D.C.Y., Sawamura, S., Taniguchi, Y., Koga, Y.J.: Excess partial molar enthalpy of 1-propanol in 1-propanol–NaCl–H2O at 25°C: the effect of NaCl on molecular organization of H2O. Phys. Chem. B 103, 2981–2983 (1999)
Westh, P., Koga, Y.: Intermolecular interactions in 2-butoxyethanol–DMSO–H2O. J. Phys. Chem. 100, 433–438 (1996)
Matthew, T.P., Koga, Y.: Interactions in 1-propanol-(1,2- and 1,3-) propanediol–H2O: the effect of hydrophobic vs hydrophilic moiety on the molecular organization of H2O. J. Phys. Chem. B 106, 7090–7095 (2002)
Redlich, O.J., Kister, A.T.: Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 345–348 (1948)
Rogalsky, M., Malanowski, S.: A new equation for correlation of vapour–liquid equilibrium data of strongly non-ideal mixtures. Fluid Phase Equilib. 1, 137–152 (1977)
Abrams, D.S., Prausnitz, J.M.: Statistical thermodynamics of liquid mixtures: a new expression for the excess Gibs energy of partly or completely miscible systems. AIChE J. 21, 116–128 (1975)
Renon, H., Prausnitz, J.M.: Vapor–liquid equilibrium. XI. A new expression for the excess free energy of mixing. AIChE J. 14, 135–144 (1968)
Riddick, J.A., Bunger, W.B., Sakano, T.K.: Organic Solvents, 3rd edn. Wiley, New York (1970)
Landgren, M., McEachern, D., Olofsson, Q., Randzio, S., Sunner, S.: Evaluation of excess enthalpies from flow-calorimetric measurements of enthalpies of dilution using local approximation by polynomials. J. Chem. Thermodyn. 10, 847–854 (1978)
Checoni, R.F., Francesconi, A.Z.: Measurement and correlation of excess molar enthalpy at various temperatures acetonitrile + diethylamine or s-butylamine mixtures. J. Therm. Anal. Calorim. 80, 295–301 (2005)
Venkatesulu, D., Prabhakara, M.V., Veerana, D.R.: Excess enthalpies of 2-alkoxyethanols with trichloroethylene and tetrachloroethylene at 298.15 K. Thermochim. Acta 242, 33–39 (1994)
MacMillan, W.G., Mayer, J.E.: Statistical thermodynamics of multicomponents systems. J. Chem. Phys. 48, 675–690 (1945)
Yan, X.H., Wang, Q., Chen, G.H., Han, S.J.: Azeotropes at elevated pressures for systems involving cyclohexane, 2-propanol, ethyl acetate, and butanone. J. Chem. Eng. Data 46, 1235–1238 (2001)
Wang, F.A., Chen, H.S., Zhu, J.Q., Song, J.C., Wang, Z.C.: Estimation of excess enthalpy for binary systems. J. Chem. Eng. 85, 235–243 (2002)
Acknowledgments
The authors would like to thank the Bu-Ali Sina University for providing the necessary facilities to carry out the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khanlarzadeh, K., Iloukhani, H. Measurement and Correlation of Excess Molar Enthalpy of Binary Mixtures Containing Butyl Acetate + 1-Alkanols (C1–C6) at 298.15 K. J Solution Chem 43, 1259–1269 (2014). https://doi.org/10.1007/s10953-014-0199-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-014-0199-7