Abstract
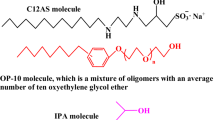
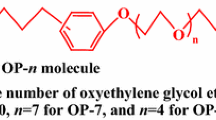
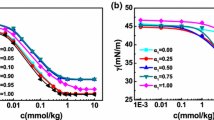
The micellization of binary mixtures of sodium dodecyl diamino sulfonate (C12AS) and nonionic octylphenol polyoxyethylene ether (10) (OP-10) was investigated in aqueous solution at a pH of about 6.0. Two techniques, UV–Vis spectroscopy using pyrene as a probe and surface tensiometry, were employed in this work to obtain information on the micellization behavior of the mixed C12AS/OP-10 system. The interaction parameters between the two components, activity coefficients in mixed micelles, compositions of mixed micelles, and thermodynamic parameters of micellization (calculated using Clint’s equation, Rubingh’s treatment based on regular solution theory, and Rodenas’s treatment considering the Gibbs–Duhem equation) were evaluated for this mixed surfactant system. The results show that the synergistic effect between C12AS and OP-10 in all mixed systems plays a vital role in the reduction of the overall critical micelle concentration (cmc) value in aqueous solution. In the process of micellization, both the steric effect of the head group and the charge density for C12AS affect the formation and stability of the mixed micelles, and the entry of a small amount of C12AS into the unconsolidated micelle of OP-10 is more favorable to the formation of the mixed micelle by promoting the reduction of the mixed micelle cmc value. Thermodynamic data show that micellization for the mixed C12AS/OP-10 system is both an enthalpy and entropy driven process.











Similar content being viewed by others
References
Rosen, M.J.: Chap. 11. Surfactants and Interfacial Phenomena, 3rd edn. Wiley, Hoboken (2004)
Ren, Z.H., Chen, D.J., Luo, Y., Huang, J.: Investigation of influencing of inorganic salt on the critical micelle concentration of sodium octylphenol polyoxyethylenated ethylsulfonate. Act. Chim. Siencia 68, 1771–1775 (2010)
Holland, P.M., Rubingh, D.N.: Nonideal multicomponent mixed micelle model. J. Phys. Chem. 87, 1984–1990 (1983)
Rodenas, E., Valiente, M., Villafruela, M.S.: Different theoretical approaches for the study of the mixed tetraethylene glycol mono-n-dodecyl etherhexadecyltrimethylammonium bromide micelles. J. Phys. Chem. 87, 4549–4554 (1999)
Hoffmann, H., Possnecker, G.: The mixing behavior of surfactants. Langmuir 10, 381–389 (1994)
Motomura, K., Yamanaka, M., Aratono, M.: Thermodynamic consideration of the mixed micelle of surfactants. Colloid Polym. Sci. 262, 948–955 (1984)
Jarek, E., Wydro, P., Warszynski, P., Paluch, M.: Surface properties of mixtures of surface-active sugar derivatives with ionic surfactants: theoretical and experimental investigations. J. Colloid Interface Sci. 293, 194–202 (2006)
Parekh, P., Varade, D., Parikh, J., Bahadur, P.: Anionic–cationic mixed surfactant systems Micellar interaction of sodium dodecyl trioxyethylene sulfate with cationic gemini surfactants. Colloids Surf. A 385, 111–120 (2011)
Cui, Z.G., Canselier, J.P.: Interfacial and aggregation properties of some anionic–cationic surfactant binary systems II. Mixed micelle formation and surface tension reduction effectiveness. Colloid Polym. Sci. 279, 259–267 (2001)
Rosen, M.J.: Synergism in mixtures containing zwitterionic surfactants. Langmuir 7, 885–888 (1991)
Haque, M.E., Das, A.R., Rakshit, A.K., Moulik, S.P.: Properties of mixed micelles of binary surfactant combinations. Langmuir 12, 4084–4089 (1996)
Lu, S.H., Wu, J., Somaundaran, P.: Micellar evolution in mixed nonionic anionic surfactant systems. J. Colloid Interface Sci. 367, 272–279 (2012)
Rosen, M.J., Murphy, D.S.: Synergism in binary mixtures of surfactants: V. Two-phase liquid–liquid systems at low surfactant concentrations. J. Colloid Interface Sci. 110, 225–232 (1986)
Das, C., Chakraborty, T., Ghosh, S., Das, B.: Mixed micellization of anionic–nonionic surfactants in aqueous media: a physicochemical study with theoretical consideration. Colloid Polym. Sci. 286, 1143–1155 (2008)
Kharitonova, T.V., Ivanova, N.I., Summ, B.D.: Intermolecular interactions in the binary systems of cationic and nonionic surfactants. Colloid J. 64, 620–631 (2002)
Ruiz, C.C., Aguiar, J.: Mixed micellization of octaoxyethylene monododecyl ether and n-alkyltrimethylammonium bromides. Colloids Surf. A 224, 221–230 (2003)
Sehgal, P., Kosaka, O., Doe, H., Otzen, D.E.: Interaction and stability of mixed micelle and monolayer of nonionic and cationic surfactant mixtures. J. Dispers. Sci. Technol. 30, 1050–1058 (2009)
Ren, Z.H., Luo, Y.: Amino sulfonate amphoteric surfactants and the process for preparing them. C.N. 101912745 A (2011)
Lange, H., Beck, K.H.: Zur mizellbildung in mischlösungen homologer und nichthomologer tenside. Kolloid Z. Z. Polym. 251, 424–431 (1973)
Clint, J.H.: Micellisation of mixed nonionic surface active agents. J. Chem. Soc. Faraday Trans. 71, 1327–1334 (1975)
Ren, Z.H., Luo, Y., Shi, D.P.: Mechanism on the interaction between amino sulfonate amphoteric surfactant and sodium dodecyl benzene sulfonate in aqueous solution. Colloids Surf. A 428, 18–24 (2013)
Crook, E.H., Trebbi, G.F., Fordyce, D.B.: Thermodynamic properties of solutions of homogeneous p, t-octylphenoxyethoxyethanols (OPE1-10). J. Phys. Chem. 68, 3592–3599 (1964)
Mehta, S.K., Bhawna, T.: Significant effect of polar head group of surfactants on the solubilization of Zein in mixed micellar (SDS–DDAB) media. Colloids Surf. B 81, 74–80 (2010)
Faustino, C.M.C., Calado, A.R.T., Garcia-Rio, L.: Mixed micelle formation between amino acid-based surfactants and phospholipids. J. Colloid Interface Sci. 359, 493–498 (2011)
Rosen, M.J.: Predicting synergism in binary mixtures of surfactants. Progr. Colloid Polym. Sci. 95, 39–47 (1994)
Hines, J.D., Thomas, R.K., Garrett, P.R., Rennie, G.K., Penfold, J.: Investigation of mixing in binary surfactant solutions by surface tension and neutron reflection: strongly interacting anionic/zwitterionic mixtures. J. Phys. Chem. B 102, 8834–8846 (1998)
Acknowledgments
Funding for this work was provided by the National Sciences Foundation of China (51304029 and 41202111), the Basic Science Research Development Fund and the Ph.D. Start-up Fund of Yangtze University, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, Z.H., Luo, Y., Zheng, Y.C. et al. Interaction Behavior Between an Amino Sulfonate Surfactant and Octylphenol Polyoxyethylene Ether (10) in Aqueous Solution. J Solution Chem 43, 853–869 (2014). https://doi.org/10.1007/s10953-014-0173-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-014-0173-4