Abstract
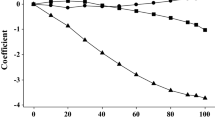
A new method for the prediction of solubility in the water–ethanol system has been devised. The method is based on the construction of equations for the prediction of partition coefficients from water to water–ethanol mixtures, based on the Abraham descriptors for compounds. Once partition coefficients have been predicted for a particular compound, only an experimental value for the solubility in ethanol is required for the prediction of solubilities in water–ethanol mixtures. Results of predictions are comparable to the Jouyban-Acree model that requires experimental solubilities in both water and ethanol.
Similar content being viewed by others
References
Yalkowsky, S., Flynn, G., Amidon, G.: Solubility of nonelectrolytes in polar solvents. J. Pharm. Sci. 61, 983–984 (1972)
Yalkowsky, S., Valvani, S., Amidon, G.: Solubility of nonelectrolytes in polar solvents. IV. Nonpolar drugs in mixed solvents. J. Pharm. Sci. 65, 1488–1493 (1976)
Li, A., Yalkowsky, S.H.: Solubility of organic solutes in ethanol/water mixtures. J. Pharm. Sci. 83, 1735–1740 (1994)
Millard, J.W., Alvarez-Nunez, F.A., Yalkowsky, S.H.: Solubilization by cosolvents. Establishing useful constants for the log-linear model. Int. J. Pharm. 245, 153–166 (2002)
Machatha, S.G., Yalkowsky, S.H.: Bilinear model for the prediction of drug solubility in ethanol/water mixtures. J. Pharm. Sci. 94, 2731–2734 (2005)
Acree, W.E. Jr., Zvaigzne, A.I.: Thermodynamic properties of nonelectrolyte solutions. Part 4. Estimation and mathematical representation of solute activity coefficients and solubilities in binary solvents using the nearly ideal binary solvent (NIBS) and modified Wilson equations. Thermochim. Acta 178, 151–161 (1991)
Matsuda, H., Kaburagi, K., Matsumoto, S., Kurihara, K., Tochigi, K., Tomono, K.: Solubilities of salicylic acid in pure solvents and binary mixtures containing cosolvent. J. Chem. Eng. Data 54, 480–484 (2009)
Pena, M.A., Reillo, A., Escalera, B., Bustamante, P.: Solubility parameter of drugs for predicting the solubility profile type of drugs within a wide range in solvent mixtures. Pharm. Nanotech. 321, 155–161 (2006)
Acree, W.E. Jr.: Mathematical representation of thermodynamic properties. Part 2. Derivation of the combined nearly ideal binary solvent (NIBS)/Redlich-Kister mathematical representation from a two-body and three-body interactional mixing model. Thermochim. Acta 198, 71–79 (1992)
Abraham, M.H.: Scales of hydrogen bonding: their construction and application to physicochemical and biochemical processes. Chem. Soc. Rev. 22, 73–83 (1993)
Abraham, M.H., Ibrahim, A., Zissimos, A.M.: The determination of sets of solute descriptors from chromatographic measurements. J. Chromatogr. A 1037, 29–47 (2004)
Jouyban, A., Soltanpour, Sh., Soltani, S., Tamizi, E., Fakhree, M.A.A., Acree, W.E. Jr.: Prediction of drug solubility in mixed solvents using computed Abraham parameters. J. Mol. Liq. 146, 82–88 (2009)
Fakhtree, M.A.A., Shayanfar, A., Acree, W.E.Jr., Jouyban, A.: Solubility of phenanthrene in binary mixtures of C1–C4 alcohols + 2-propanol and ethanol + methanol at 298.2 K. J. Chem. Eng. Data 54, 1405–1408 (2009)
Abraham, M.H., Acree, W.E. Jr., Cometto-Muñiz, J.E.: Partition of compounds from water and air into amides. New J. Chem. 33, 2034–2043 (2009) (2009)
Flanagan, K.B., Hoover, K.C., Garza, O., Hizon, A., Soto, T., Villegas, N., Acree, W.E. Jr., Abraham, M.H.: Mathematical correlation of 1-chloroanthraquinone solubilities in organic solvents with the Abraham solubility parameter model. Phys. Chem. Liq. 44, 377–386 (2006)
Blake-Taylor, B.H., Delean, V.H., Acree, W.E. Jr., Abraham, M.H.: Mathematical correlation of salicylamide solubilities in organic solvents with the Abraham solvation parameter model. Phys. Chem. Liq. 45, 389–398 (2007)
Abraham, M.H., Smith, R.E., Luchtefeld, R., Boorem, A.J., Luo, R., Acree, W.E. Jr.: Prediction of solubility of drugs and other compounds in organic solvents. J. Pharm. Sci. 99, 1500–1515 (2010)
Yamamoto, H., Ichikawa, K., Tokunaga, J.: Solubility of helium in methanol + water, ethanol + water, 1-propanol + water and 2-propanol + water solutions at 25 °C. J. Chem. Eng. Data 39, 155–157 (1994)
Abraham, M.H., Grellier, P.L., McGill, R.A.: A quantitative measure of solvent solvophobic effect. J. Chem. Soc. Perkin Trans. II 339–345 (1988)
Young, C.L.: Hydrogen and Deuterium. Solubility Data Series, vol. 5/6. Pergamon Press, Oxford (1981)
Tokunaga, J.: Solubilities of oxygen, nitrogen and carbon dioxide in aqueous alcohol solutions. J. Chem. Eng. Data 20, 41–46 (1975)
Winstein, S., Fainberg, A.H.: Correlation of solvolysis rates. IV. Solvent effects on enthalpy and entropy of activation for solvolysis of tert-butyl chloride. J. Am. Chem. Soc. 79, 5937–5950 (1957)
Starobinets, G.L., Rakhman’ko, E.M., Leshchev, S.M.: Medium effects on the partition of organic non-electrolytes between mixed water–ethanol media and octane. Russ. J. Phys. Chem. 52, 2284–2287 (1978)
Seidell, A.: Solubilities of Organic Compounds, 3rd edn., vol. II. Van Nostrand, New York (1941)
Zhou, B., Cai, W., Zou, L.: Thermodynamic functions for transfer of anthracene from water to (water + alcohol) mixtures at 298.15 K. J. Chem. Eng. Data 48, 742–745 (2003)
Abraham, M.H.: Unpublished work on the solubility of benzyl chloride (2010)
Li, A., Andren, A.A.: Solubility of polychlorinated biphenyls in water/alcohol mixtures. 1. Experimental data. Environ. Sci. Technol. 28, 47–52 (1994)
Xiao, M., Yan, W., Zhang, Z.: Solubilities of apigenin in ethanol + water at different temperatures. J. Chem. Eng. Data 55, 3346–3348 (2010)
Peng, B., Yan, W.: Solubility of luteolin in ethanol + water mixed solvents at different temperatures. J. Chem. Eng. Data 55, 583–585 (2010)
Peng, B., Li, R., Yan, W.: Solubility of rutin in ethanol + water at (273.15 to 323.15) K. J. Chem. Eng. Data 54, 1378–1381 (2009)
Bose, K., Kundu, K.K.: Thermodynamics of transfer of p-nitroaniline from water to alcohol–water mixtures at 25 °C and the structure of water in these mixtures. Can. J. Chem. 55, 3961–3965 (1977)
Peña, M.A., Reillo, A., Escalera, B., Bustamente, P.: Solubility parameter of drugs for predicting the solubility profile type within a wide polarity range in solvent mixtures. Int. J. Pharm. 321, 155–161 (2006)
Gantiva, M., Yurquina, A., Martinez, F.: Solution thermodynamics of ketoprofen in ethanol + water cosolvent mixtures. J. Chem. Eng. Data 55, 113–118 (2010)
Perez-Camino, M., Sanchez, E., Balon, M., Maestre, A.: Thermodynamic functions for the transfer of 1-naphthoic acid from water to mixed aqueous solvents at 298 K. J. Chem. Soc. Faraday Trans. I 81, 1555–1561 (1985)
Jouyban, A., Chan, H.K., Romero, S., Khoubnasabjafari, M., Bustamante, P.: Solubility prediction in water–ethanol mixtures based on the excess free energy approach using a minimum number of experimental data. Pharmazie 59, 117–120 (2004)
Lukavenko, O.N., Eltsov, S.V., Grigorovich, A.V., Mchedlov-Petrossyan, N.O.: Solubility and fluorescence lifetime of 2,5-diphenyloxazole and 1,4-bis(5-phenyloxazolyl-2)benzene in water–ethanol and water-acetone systems. J. Mol. Liq. 145, 167–172 (2009)
Liu, Y., Wang, J., Wang, X., Liu, P., Pang, F.: Solubility of valsartan in different organic solvents and ethanol + water binary mixtures from (278.15 to 313.15 ) K. J. Chem. Eng. Data 54, 986–988 (2009)
Zhao, G., Yan, W.: Experimental determination of solubilities of betulin in acetone + water and ethanol + water mixed solvents at T=(278.2,288.2,298.2,308.2, and 318.2) K. J. Chem. Eng. Data 52, 2365–2367 (2007)
Breon, T.L., Paruta, A.N.: Solubility profiles for several barbiturates in hydroalcoholic mixtures. J. Pharm. Sci. 59, 1306–1313 (1970)
Manzo, R.H., Ahumada, A.A., Luna, E.: Effects of medium on solubility III: hydrophilic-lipophilic character exhibited by some functional groups having oxygen or nitrogen in ethanol–water. J. Pharm. Sci. 73, 1094–1097 (1984)
Manzo, R.H., Ahumada, A.A., Luna, E.: Effects of medium on solubility IV: comparison of the hydrophilic-lipophilic character exhibited by functional groups in ethanol–water. J. Pharm. Sci. 73, 1869–1871 (1984)
Ali, H.S.M., York, P., Blagden, N., Soltanpour, S., Acree, W.E. Jr., Jouyban, A.: Solubility of budesonide, hydrocortisone, and prednisolone in ethanol + water mixtures at 298.2 K. J. Chem. Eng. Data 55, 578–582 (2010)
Desai, K.G.H., Kulkarni, A.R., Aminabhavi, T.M.: Solubility of rofecoxib in the presence of methanol, ethanol and sodium lauryl sulfate at (298.15, 301.15, and 308.15) K. J. Chem. Eng. Data 48, 942–945 (2003)
Liu, C., Desai, K.G.H., Liu, C.: Solubility of valdecoxib in the presence of ethanol and sodium lauryl sulfate at (298.15, 303.15, and 308.15) K. J. Chem. Eng. Data 49, 1847–1850 (2004)
Paruta, A.N.: Solubility profiles for antipyrine and aminopyrine in hydroalcoholic solutions. J. Pharm. Sci. 56, 1565–1569 (1967)
Abraham, M.H., Acree, W.E. Jr.: Equations for the transfer of neutral molecules and ionic species from water to organic phases. J. Org. Chem. 75, 1006–1015 (2010)
Franks, F.: The properties of aqueous solutions of non-electrolytes. In: Franks, F. (ed.) Physico-Chemical Processes in Mixed Aqueous Solvents. Heinemann, London (1967)
Sprunger, L.M., Achi, S.S., Pointer, R., Blake-Taylor, B.H., Acree, W.E. Jr., Abraham, M.H.: Development of Abraham model correlations for solvation characteristics of linear alcohols. Fluid Phase Equilib. 286, 170–174 (2009)
Li, A., Pinsuwan, S., Yalkowsky, S.H.: Estimation of solubility of organic compounds in 1-octanol. Ind. Eng. Chem. 34, 915–920 (1995)
Hilal, S.H., Karickhoff, S.W., Carreira, L.A.: Prediction of the solubility, activity coefficient and liquid/liquid partition coefficient of organic compounds. QSAR & Comb. Sci. 23, 709–720 (2004)
Eckert, F.: Prediction of solubility with COSMO-RS. In: Letcher, T.M. (ed.) Developments and Applications in Solubility. Royal Society of Chemistry, Cambridge (2007)
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abraham, M.H., Acree, W.E. Partition Coefficients and Solubilities of Compounds in the Water–Ethanol Solvent System. J Solution Chem 40, 1279–1290 (2011). https://doi.org/10.1007/s10953-011-9719-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-011-9719-x